
Emtricitabine, with trade name Emtriva, is a nucleoside reverse-transcriptase inhibitor (NRTI) for the prevention and treatment of HIV infection in adults and children. In 2019, it was the 494th most commonly prescribed medication in the United States, with more than 3 thousand prescriptions.
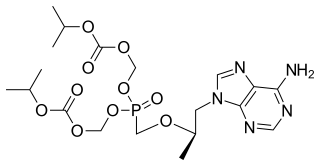
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder.
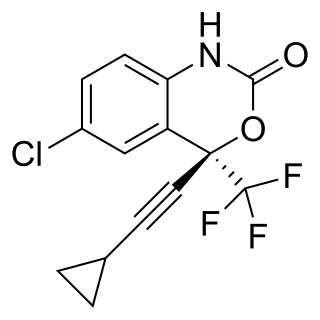
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth.

Emtricitabine/tenofovir, sold under the brand name Truvada among others, is a fixed-dose combination antiretroviral medication used to treat and prevent HIV/AIDS. It contains the antiretroviral medications emtricitabine and tenofovir disoproxil. For treatment, it must be used in combination with other antiretroviral medications. For prevention before exposure, in those who are at high risk, it is recommended along with safer sex practices. It does not cure HIV/AIDS. Emtricitabine/tenofovir is taken by mouth.

Pre-exposure prophylaxis for HIV prevention, commonly known as PrEP, is a form of pre-exposure prophylaxis to prevent HIV infection, the cause of HIV/AIDS.

Efavirenz/emtricitabine/tenofovir, sold under the brand name Atripla among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains efavirenz, emtricitabine, and tenofovir disoproxil. It can be used by itself or together with other antiretroviral medications. It is taken by mouth.

The HIV Prevention Trials Network (HPTN) is a worldwide collaborative clinical trials network that brings together investigators, ethicists, community and other partners to develop and test the safety and efficacy of interventions designed to prevent the acquisition and transmission of HIV. HPTN studies evaluate new HIV prevention interventions and strategies in populations and geographical regions that bear a disproportionate burden of infection. The HPTN is committed to the highest ethical standards for its clinical trials and recognizes the importance of community engagement in all phases of the research process.
Integrase inhibitors (INIs) are a class of antiretroviral drug designed to block the action of integrase, a viral enzyme that inserts the viral genome into the DNA of the host cell. Since integration is a vital step in retroviral replication, blocking it can halt further spread of the virus. Integrase inhibitors were initially developed for the treatment of HIV infection, but have been applied to other retroviruses. The class of integrase inhibitors called integrase strand transfer inhibitors (INSTIs) are in established medical use. Other classes, such as integrase binding inhibitors (INBIs), are still experimental.

Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.

iPrEx was a phase III clinical trial to determine whether the antiretroviral medication emtricitabine/tenofovir could safely and effectively prevent HIV acquisition through sex in men who have sex with men and transgender women. iPrEx was the first human study of an HIV prevention strategy known as pre-exposure prophylaxis, or PrEP.

Dolutegravir (DTG), sold under the brand name Tivicay, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. It is taken by mouth.
Emtricitabine/rilpivirine/tenofovir is a fixed-dose combination of antiretroviral drugs for the treatment of HIV/AIDS. The drug was co-developed by Gilead Sciences and Johnson & Johnson's Tibotec division and was approved by the Food and Drug Administration in August 2011, and by the European Medicines Agency in November 2011, for patients who have not previously been treated for HIV. It is available as a once-a-day single tablet.
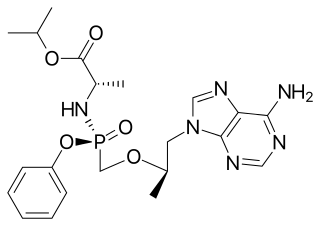
Tenofovir alafenamide, sold under the brand name Vemlidy, is an antiviral medication used against hepatitis B and HIV. It is used for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease and is given in combination with other medications for the prevention and treatment of HIV. It is taken by mouth.
Elvitegravir/cobicistat/emtricitabine/tenofovir, sold under the brand name Stribild, also known as the Quadpill, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. Elvitegravir, emtricitabine and tenofovir disoproxil directly suppress viral reproduction. Cobicistat increases the effectiveness of the combination by inhibiting the liver and gut wall enzymes that metabolize elvitegravir. It is taken by mouth.

Abacavir/dolutegravir/lamivudine, sold under the brand name Triumeq among others, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It is a combination of three medications with different and complementary mechanisms of action: abacavir, dolutegravir and lamivudine.
HPTN 083 is a 2016 clinical trial which compares cabotegravir injections with oral use of Emtricitabine/tenofovir as pre-exposure prophylaxis ("PrEP") for prevention of HIV/AIDS.

Bictegravir/emtricitabine/tenofovir alafenamide, sold under the brand name Biktarvy, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. One tablet, taken orally once daily, contains 50 mg bictegravir, 200 mg emtricitabine, and 25 mg tenofovir alafenamide. It was approved for use in the United States in February 2018, and for use in the European Union in June 2018.
Dolutegravir/lamivudine, sold under the brand name Dovato, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains dolutegravir, as the salt, an integrase strand transfer inhibitor (INSTI), and lamivudine, a nucleoside analogue reverse transcriptase inhibitor (NRTI). It is taken by mouth.

Cabotegravir/rilpivirine, sold under the brand name Cabenuva, is a co-packaged antiretroviral medication for the treatment of HIV/AIDS. It contains cabotegravir and rilpivirine in a package with two separate injection vials.

Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS. It is taken by mouth or by subcutaneous injection.

















