Thalassemias are inherited blood disorders that result in abnormal hemoglobin. Symptoms depend on the type of thalassemia and can vary from none to severe. Often there is mild to severe anemia as thalassemia can affect the production of red blood cells and also affect how long the red blood cells live. Symptoms of anemia include feeling tired and having pale skin. Other symptoms of thalassemia include bone problems, an enlarged spleen, yellowish skin, pulmonary hypertension, and dark urine. Slow growth may occur in children. Symptoms and presentations of thalassemia can change over time.

Chronic myelogenous leukemia (CML), also known as chronic myeloid leukemia, is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes and their precursors is found; characteristic increase in basophils is clinically relevant. It is a type of myeloproliferative neoplasm associated with a characteristic chromosomal translocation called the Philadelphia chromosome.

Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple tyrosine kinases such as CSF1R, ABL, c-KIT, FLT3, and PDGFR-β. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome–positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome.
Fetal hemoglobin, or foetal haemoglobin is the main oxygen carrier protein in the human fetus. Hemoglobin F is found in fetal red blood cells, and is involved in transporting oxygen from the mother's bloodstream to organs and tissues in the fetus. It is produced at around 6 weeks of pregnancy and the levels remain high after birth until the baby is roughly 2–4 months old. Hemoglobin F has a different composition than adult forms of hemoglobin, allowing it to bind oxygen more strongly; this in turn enables the developing fetus to retrieve oxygen from the mother's bloodstream, which occurs through the placenta found in the mother's uterus.

Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma. It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.

In hematology, thrombocythemia is a condition of high platelet (thrombocyte) count in the blood. Normal count is in the range of 150×109 to 450×109 platelets per liter of blood, but investigation is typically only considered if the upper limit exceeds 750×109/L.

In hematology, essential thrombocythemia (ET) is a rare chronic blood cancer characterised by the overproduction of platelets (thrombocytes) by megakaryocytes in the bone marrow. It may, albeit rarely, develop into acute myeloid leukemia or myelofibrosis. It is one of the blood cancers wherein the bone marrow produces too many white or red blood cells, or platelets.
Primary myelofibrosis (PMF) is a rare bone marrow blood cancer. It is classified by the World Health Organization (WHO) as a type of myeloproliferative neoplasm, a group of cancers in which there is activation and growth of mutated cells in the bone marrow. This is most often associated with a somatic mutation in the JAK2, CALR, or MPL genes. In PMF, the bony aspects of bone marrow are remodeled in a process called osteosclerosis; in addition, fibroblast secrete collagen and reticulin proteins that are collectively referred to as (fibrosis). These two pathological processes compromise the normal function of bone marrow resulting in decreased production of blood cells such as erythrocytes, granulocytes and megakaryocytes, the latter cells responsible for the production of platelets.
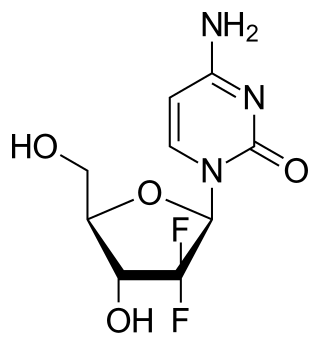
Gemcitabine, sold under the brand name Gemzar, among others, is a chemotherapy medication used to treat cancers. It is used to treat testicular cancer, breast cancer, ovarian cancer, non-small cell lung cancer, pancreatic cancer, and bladder cancer. It is administered by intravenous infusion. It acts against neoplastic growth, and it inhibits the replication of Orthohepevirus A, the causative agent of Hepatitis E, through upregulation of interferon signaling.

Myeloproliferative neoplasms (MPNs) are a group of rare blood cancers in which excess red blood cells, white blood cells or platelets are produced in the bone marrow. Myelo refers to the bone marrow, proliferative describes the rapid growth of blood cells and neoplasm describes that growth as abnormal and uncontrolled.

Mercaptopurine (6-MP), sold under the brand name Purinethol among others, is a medication used for cancer and autoimmune diseases. Specifically it is used to treat acute lymphocytic leukemia (ALL), acute promyelocytic leukemia (APL), Crohn's disease, and ulcerative colitis. For acute lymphocytic leukemia it is generally used with methotrexate. It is taken orally.
The era of cancer chemotherapy began in the 1940s with the first use of nitrogen mustards and folic acid antagonist drugs. The targeted therapy revolution has arrived, but many of the principles and limitations of chemotherapy discovered by the early researchers still apply.

Azacitidine, sold under the brand name Vidaza among others, is a medication used for the treatment of myelodysplastic syndrome, myeloid leukemia, and juvenile myelomonocytic leukemia. It is a chemical analog of cytidine, a nucleoside in DNA and RNA. Azacitidine and its deoxy derivative, decitabine were first synthesized in Czechoslovakia as potential chemotherapeutic agents for cancer.

Melphalan, sold under the brand name Alkeran among others, is a chemotherapy medication used to treat multiple myeloma; malignant lymphoma; lymphoblastic and myeloblastic leukemia; childhood neuroblastoma; ovarian cancer; mammary adenocarcinoma; and uveal melanoma. It is taken by mouth or by injection into a vein.

Anagrelide is a drug used for the treatment of essential thrombocytosis, or overproduction of blood platelets. It also has been used in the treatment of chronic myeloid leukemia.

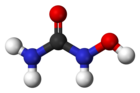
Sodium phenylbutyrate, sold under the brand name Buphenyl among others, is a salt of an aromatic fatty acid, 4-phenylbutyrate (4-PBA) or 4-phenylbutyric acid. The compound is used to treat urea cycle disorders, because its metabolites offer an alternative pathway to the urea cycle to allow excretion of excess nitrogen.
Hereditary persistence of fetal hemoglobin (HPFH) is a benign condition in which increased fetal hemoglobin production continues well into adulthood, disregarding the normal shutoff point after which only adult-type hemoglobin should be produced.

Sickle cell disease (SCD), one of the hemoglobinopathies, is a group of blood disorders typically inherited. The most common type is known as sickle cell anaemia. It results in an abnormality in the oxygen-carrying protein haemoglobin found in red blood cells. This leads to a rigid, sickle-like shape under certain circumstances. Problems in sickle cell disease typically begin around 5 to 6 months of age. A number of health problems may develop, such as attacks of pain, anemia, swelling in the hands and feet, bacterial infections, and stroke. Long-term pain may develop as people get older. The average life expectancy in the developed world is 40 to 60 years.
VAMP regimen or VAMP chemotherapy is a four-drug combination chemotherapy regimen, used today in the treatment of Hodgkin lymphoma. It was one of the earliest combination chemotherapy regimens, originally developed as a treatment for childhood leukemia by a group of researchers at the National Cancer Institute led by Emil Frei and Emil Freireich. The first clinical trial of VAMP began in 1961. Because it was the first time that four chemotherapeutic agents were used at once, the trial was highly controversial at its time. Although new combination chemotherapy regimens have replaced the use of VAMP in the treatment of childhood leukemia, VAMP is considered an important precursor to modern treatments, confirming the effectiveness of combination chemotherapy and leading to the use of combination chemotherapy regimens to treat other types of cancer.
Sickle cell-beta thalassemia is an inherited blood disorder. The disease may range in severity from being relatively benign and like sickle cell trait to being similar to sickle cell disease.