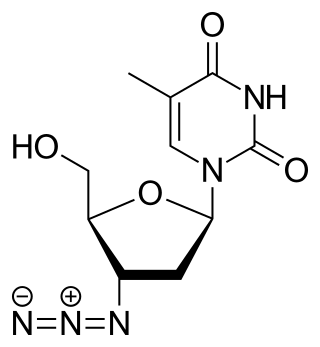
Zidovudine (ZDV), also known as azidothymidine (AZT), was the first antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same-sex and opposite-sex partners so long as the HIV-positive partner maintains an undetectable viral load.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Didanosine, sold under the brand name Videx, is a medication used to treat HIV/AIDS. It is used in combination with other medications as part of highly active antiretroviral therapy (HAART). It is of the reverse-transcriptase inhibitor class.

Abacavir, sold under the brand name Ziagen among others, is a medication used to treat HIV/AIDS. Similar to other nucleoside analog reverse-transcriptase inhibitors (NRTIs), abacavir is used together with other HIV medications, and is not recommended by itself. It is taken by mouth as a tablet or solution and may be used in children over the age of three months.

Emtricitabine, with trade name Emtriva, is a nucleoside reverse-transcriptase inhibitor (NRTI) for the prevention and treatment of HIV infection in adults and children. In 2019, it was the 494th most commonly prescribed medication in the United States, with more than 3 thousand prescriptions.

Nevirapine (NVP), sold under the brand name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1. It is generally recommended for use with other antiretroviral medications. It may be used to prevent mother to child spread during birth but is not recommended following other exposures. It is taken by mouth.
Integrase inhibitors (INIs) are a class of antiretroviral drug designed to block the action of integrase, a viral enzyme that inserts the viral genome into the DNA of the host cell. Since integration is a vital step in retroviral replication, blocking it can halt further spread of the virus. Integrase inhibitors were initially developed for the treatment of HIV infection, but have been applied to other retroviruses. The class of integrase inhibitors called integrase strand transfer inhibitors (INSTIs) are in established medical use. Other classes, such as allosteric integrase inhibitors (ALLINIs) or integrase binding inhibitors (INBIs), are still experimental.
Maraviroc, sold under the brand names Selzentry (US) and Celsentri (EU), is an antiretroviral medication used to treat HIV infection. It is taken by mouth. It is in the CCR5 receptor antagonist class.

Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.

Dolutegravir (DTG), sold under the brand name Tivicay, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. It is taken by mouth.
The first human immunodeficiency virus (HIV) case was reported in the United States in the early 1980s. Many drugs have been discovered to treat the disease but mutations in the virus and resistance to the drugs make development difficult. Integrase is a viral enzyme that integrates retroviral DNA into the host cell genome. Integrase inhibitors are a new class of drugs used in the treatment of HIV. The first integrase inhibitor, raltegravir, was approved in 2007 and other drugs were in clinical trials in 2011.

Fostemsavir, sold under the brand name Rukobia, is an antiretroviral medication for adults living with HIV/AIDS who have tried multiple HIV medications and whose HIV infection cannot be successfully treated with other therapies because of resistance, intolerance or safety considerations.

Abacavir/dolutegravir/lamivudine, sold under the brand name Triumeq among others, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It is a combination of three medications with different and complementary mechanisms of action: abacavir, dolutegravir and lamivudine.

Bictegravir/emtricitabine/tenofovir alafenamide, sold under the brand name Biktarvy, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. One tablet, taken orally once daily, contains 50 mg bictegravir, 200 mg emtricitabine, and 25 mg tenofovir alafenamide.
Doravirine/lamivudine/tenofovir, sold under the brand name Delstrigo, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains doravirine, lamivudine, and tenofovir disoproxil. It is taken by mouth.
Dolutegravir/lamivudine, sold under the brand name Dovato, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains dolutegravir, as the salt, an integrase strand transfer inhibitor (INSTI), and lamivudine, a nucleoside analogue reverse transcriptase inhibitor (NRTI). It is taken by mouth.
Daria J Hazuda is a biochemist known for discovering the first HIV Integrase Strand Transfer Inhibitors (InSTIs) and leading the development of the first HIV integrase inhibitor to gain FDA approval, Isentress (raltegravir). Her lab also determined these inhibitors' mechanism of action and ways the virus could develop resistance to them. Hazuda has also performed extensive research and led the development of antivirals for Hepatitis C Virus (HCV) including Elbasvir and Grazoprevir. She currently serves as Vice President for Infectious Diseases Discovery for Merck and Chief Scientific Officer of their MRL Cambridge Exploratory Science Center.

Cabotegravir/rilpivirine, sold under the brand name Cabenuva, is a co-packaged antiretroviral medication for the treatment of HIV/AIDS. It contains cabotegravir and rilpivirine in a package with two separate injection vials.

Charles Williams Flexner is an American physician, clinical pharmaceutical scientist, academic, author and researcher. He is a Professor of Medicine at the Johns Hopkins University School of Medicine.
















