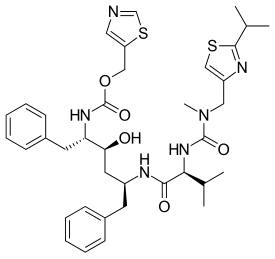
Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

Atazanavir, sold under the brand name Reyataz among others, is an antiretroviral medication used to treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth.

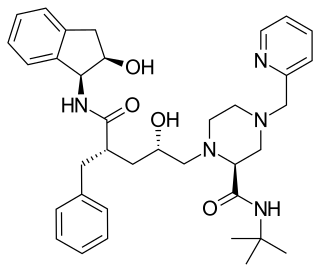
Saquinavir, sold under the brand name Invirase among others, is an antiretroviral medication used together with other medications to treat or prevent HIV/AIDS. Typically it is used with ritonavir or lopinavir/ritonavir to increase its effect. It is taken by mouth.

Tipranavir (TPV), or tipranavir disodium, is a nonpeptidic protease inhibitor (PI) manufactured by Boehringer Ingelheim under the trade name AptivusAP-tiv-əs. It is administered with ritonavir in combination therapy to treat HIV infection.
Indinavir is a protease inhibitor used as a component of highly active antiretroviral therapy to treat HIV/AIDS. It is soluble white powder administered orally in combination with other antiviral drugs. The drug prevents protease from functioning normally. Consequently, HIV viruses cannot reproduce, causing a decrease in the viral load. Commercially sold indinavir is indinavir anhydrous, which is indinavir with an additional amine in the hydroxyethylene backbone. This enhances its solubility and oral bioavailability, making it easier for users to intake. It was synthetically produced for the purpose of inhibiting the protease in the HIV virus.

Fosamprenavir (FPV), sold under the brand names Lexiva and Telzir, is a medication used to treat HIV/AIDS. It is a prodrug of the protease inhibitor and antiretroviral drug amprenavir. It is marketed by ViiV Healthcare as the calcium salt.
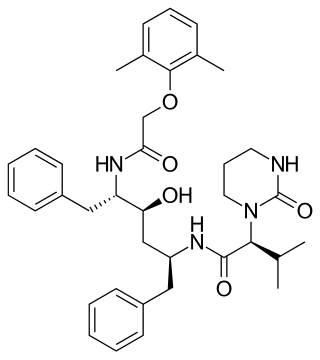
Lopinavir is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir (lopinavir/ritonavir).

Umifenovir, sold under the brand name Arbidol, is an antiviral medication for the treatment of influenza and COVID infections used in Russia and China. The drug is manufactured by Pharmstandard. It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.

Darunavir (DRV), sold under the brand name Prezista among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It is often used with low doses of ritonavir or cobicistat to increase darunavir levels. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth once to twice a day.

Elvitegravir (EVG) is an integrase inhibitor used to treat HIV infection. It was developed by the pharmaceutical company Gilead Sciences, which licensed EVG from Japan Tobacco in March 2008. The drug gained approval by the U.S. Food and Drug Administration on August 27, 2012, for use in adult patients starting HIV treatment for the first time as part of the fixed dose combination known as Stribild. On September 24, 2014, the FDA approved Elvitegravir as a single pill formulation under the trade name Vitekta. On November 5, 2015, the FDA approved the drug for use in patients affected with HIV-1 as a part of a second fixed dose combination pill known as Genvoya.

Cobicistat, sold under the brand name Tybost, is a medication for use in the treatment of human immunodeficiency virus infection (HIV/AIDS). Its major mechanism of action is through the inhibition of human CYP3A proteins.

Lopinavir/ritonavir (LPV/r), sold under the brand name Kaletra among others, is a fixed-dose combination antiretroviral medication for the treatment and prevention of HIV/AIDS. It combines lopinavir with a low dose of ritonavir. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth as a tablet, capsule, or solution.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.

The 3C-like protease (3CLpro) or main protease (Mpro), formally known as C30 endopeptidase or 3-chymotrypsin-like protease, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.
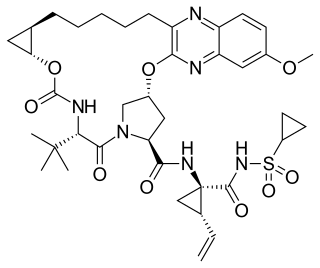
Grazoprevir is a drug approved for the treatment of hepatitis C. It was developed by Merck and completed Phase III trials, used in combination with the NS5A replication complex inhibitor elbasvir under the trade name Zepatier, either with or without ribavirin.

Drug repositioning is the repurposing of an approved drug for the treatment of a different disease or medical condition than that for which it was originally developed. This is one line of scientific research which is being pursued to develop safe and effective COVID-19 treatments. Other research directions include the development of a COVID-19 vaccine and convalescent plasma transfusion.

COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research, with 419 potential COVID-19 drugs in clinical trials, as of April 2021.
The treatment and management of COVID-19 combines both supportive care, which includes treatment to relieve symptoms, fluid therapy, oxygen support as needed, and a growing list of approved medications. Highly effective vaccines have reduced mortality related to SARS-CoV-2; however, for those awaiting vaccination, as well as for the estimated millions of immunocompromised persons who are unlikely to respond robustly to vaccination, treatment remains important. Some people may experience persistent symptoms or disability after recovery from the infection, known as long COVID, but there is still limited information on the best management and rehabilitation for this condition.

Nirmatrelvir is an antiviral medication developed by Pfizer which acts as an orally active 3C-like protease inhibitor. It is part of a nirmatrelvir/ritonavir combination used to treat COVID-19 and sold under the brand name Paxlovid.

Nirmatrelvir/ritonavir, sold under the brand name Paxlovid, is a co-packaged medication used as a treatment for COVID‑19. It contains the antiviral medications nirmatrelvir and ritonavir and was developed by Pfizer. Both components are protease inhibitors: nirmatrelvir inhibits SARS-CoV-2 main protease, while ritonavir inhibits HIV-1 protease, and is additionally a strong CYP3A inhibitor. It is taken by mouth.