
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.

Enfuvirtide (INN), sold under the brand name Fuzeon, is an HIV fusion inhibitor, the first of a class of antiretroviral drugs used in combination therapy for the treatment of AIDS/HIV.

C-C chemokine receptor type 5, also known as CCR5 or CD195, is a protein on the surface of white blood cells that is involved in the immune system as it acts as a receptor for chemokines.

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.

C-X-C chemokine receptor type 4 (CXCR-4) also known as fusin or CD184 is a protein that in humans is encoded by the CXCR4 gene. The protein is a CXC chemokine receptor.

Envelope glycoprotein GP120 is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1984. The 120 in its name comes from its molecular weight of 120 kDa. Gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. These receptors are DC-SIGN, Heparan Sulfate Proteoglycan and a specific interaction with the CD4 receptor, particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and gp41 that lead to the fusion of the viral membrane with the host cell membrane. Binding to CD4 is mainly electrostatic although there are van der Waals interactions and hydrogen bonds.
A co-receptor is a cell surface receptor that binds a signalling molecule in addition to a primary receptor in order to facilitate ligand recognition and initiate biological processes, such as entry of a pathogen into a host cell.

Gp41 also known as glycoprotein 41 is a subunit of the envelope protein complex of retroviruses, including human immunodeficiency virus (HIV). Gp41 is a transmembrane protein that contains several sites within its ectodomain that are required for infection of host cells. As a result of its importance in host cell infection, it has also received much attention as a potential target for HIV vaccines.
HIV tropism refers to the cell type in which the human immunodeficiency virus (HIV) infects and replicates. HIV tropism of a patient's virus is measured by the Trofile assay.
Sulfatide, also known as 3-O-sulfogalactosylceramide, SM4, or sulfated galactocerebroside, is a class of sulfolipids, specifically a class of sulfoglycolipids, which are glycolipids that contain a sulfate group. Sulfatide is synthesized primarily starting in the endoplasmic reticulum and ending in the Golgi apparatus where ceramide is converted to galactocerebroside and later sulfated to make sulfatide. Of all of the galactolipids that are found in the myelin sheath, one fifth of them are sulfatide. Sulfatide is primarily found on the extracellular leaflet of the myelin plasma membrane produced by the oligodendrocytes in the central nervous system and in the Schwann cells in the peripheral nervous system. However, sulfatide is also present on the extracellular leaflet of the plasma membrane of many cells in eukaryotic organisms.
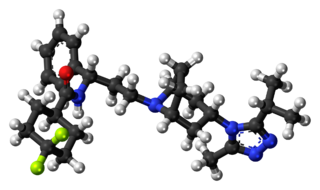
Maraviroc, sold under the brand names Selzentry (US) and Celsentri (EU), is an antiretroviral medication used to treat HIV infection. It is taken by mouth. It is in the CCR5 receptor antagonist class.
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
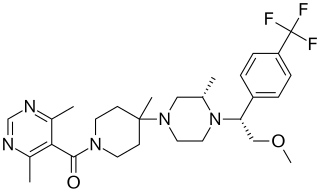
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.
CD4 immunoadhesin is a recombinant fusion protein consisting of a combination of CD4 and the fragment crystallizable region, similarly known as immunoglobulin. It belongs to the antibody (Ig) gene family. CD4 is a surface receptor for human immunodeficiency virus (HIV). The CD4 immunoadhesin molecular fusion allow the protein to possess key functions from each independent subunit. The CD4 specific properties include the gp120-binding and HIV-blocking capabilities. Properties specific to immunoglobulin are the long plasma half-life and Fc receptor binding. The properties of the protein means that it has potential to be used in AIDS therapy as of 2017. Specifically, CD4 immunoadhesin plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC) towards HIV-infected cells. While natural anti-gp120 antibodies exhibit a response towards uninfected CD4-expressing cells that have a soluble gp120 bound to the CD4 on the cell surface, CD4 immunoadhesin, however, will not exhibit a response. One of the most relevant of these possibilities is its ability to cross the placenta.
Leronlimab is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of COVID-19, triple negative breast cancer, and HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. In 2020 CytoDyn submitted a fast-track biologics license application for treatment of CCR5-tropic HIV-1 Infection.
2F5 is a broadly neutralizing human monoclonal antibody (mAb) that has been shown to bind to and neutralize HIV-1 in vitro, making it a potential candidate for use in vaccine synthesis. 2F5 recognizes an epitope in the membrane-proximal external region (MPER) of HIV-1 gp41. 2F5 then binds to this epitope and its constant region interacts with the viral lipid membrane, which neutralizes the virus.
CCR5 receptor antagonists are a class of small molecules that antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the process by which HIV, the virus that causes AIDS, enters cells. Hence antagonists of this receptor are entry inhibitors and have potential therapeutic applications in the treatment of HIV infections.

VIR-576 is an experimental drug that is under clinical trials for the treatment of HIV-1 infections. VIR-576 is synthetic peptide that binds to HIV-1's hydrophobic fusion peptide gp41, preventing the virus from inserting itself into a host cell's membrane to initiate an infection. This drug is a synthesized variant of a highly specific natural entry inhibitor designated as VIRIP.
A small proportion of humans show partial or apparently complete innate resistance to HIV, the virus that causes AIDS. The main mechanism is a mutation of the gene encoding CCR5, which acts as a co-receptor for HIV. It is estimated that the proportion of people with some form of resistance to HIV is under 10%.













