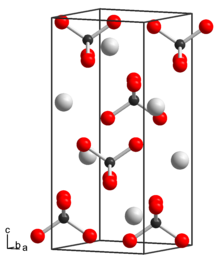
Technetium is a chemical element; it has symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide. By virtue of its radioactive decay, it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, experiments that use radioisotope tracers are sometimes called radioisotope feeding experiments.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

A technetium-99m generator, or colloquially a technetium cow or moly cow, is a device used to extract the metastable isotope 99mTc of technetium from a decaying sample of molybdenum-99. 99Mo has a half-life of 66 hours and can be easily transported over long distances to hospitals where its decay product technetium-99m is extracted and used for a variety of nuclear medicine diagnostic procedures, where its short half-life is very useful.
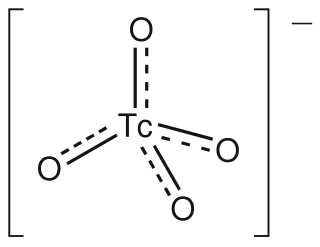
The pertechnetate ion is an oxyanion with the chemical formula TcO−
4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope which is commonly used in nuclear medicine in several nuclear scanning procedures.

Technetium(VII) oxide is the chemical compound with the formula Tc2O7. This yellow volatile solid is a rare example of a molecular binary metal oxide, the other examples being RuO4, OsO4, and the unstable Mn2O7. It adopts a centrosymmetric corner-shared bi-tetrahedral structure in which the terminal and bridging Tc−O bonds are 167pm and 184 pm respectively and the Tc−O−Tc angle is 180°.

Sodium pertechnetate is the inorganic compound with the formula NaTcO4. This colourless salt contains the pertechnetate anion, TcO−
4 that has slightly distorted tetrahedron symmetry both at 296 K and at 100 K while the coordination polyhedron of the sodium cation is different from typical for scheelite structure. The radioactive 99m
Tc
O−
4 anion is an important radiopharmaceutical for diagnostic use. The advantages to 99m
Tc
include its short half-life of 6 hours and the low radiation exposure to the patient, which allow a patient to be injected with activities of more than 30 millicuries. Na[99m
Tc
O
4] is a precursor to a variety of derivatives that are used to image different parts of the body.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.

Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99, symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world.
Technetium-99 (99Tc) is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting beta particles, but no gamma rays. It is the most significant long-lived fission product of uranium fission, producing the largest fraction of the total long-lived radiation emissions of nuclear waste. Technetium-99 has a fission product yield of 6.0507% for thermal neutron fission of uranium-235.

Pertechnetic acid (HTcO4) is a compound of technetium that is produced by reacting technetium(VII) oxide (Tc2O7) with water or strong oxidizing acids, such as nitric acid, concentrated sulfuric acid or aqua regia. The dark red hygroscopic substance is a strong acid, with a pKa of 0.32, as such it exists almost entirely as the pertechnetate ion in aqueous solution. The red color in solution is thought to be due to the formation of the polyoxometallate Tc20O4−68.
ATC code V09Diagnostic radiopharmaceuticals is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup V09 is part of the anatomical group V Various.
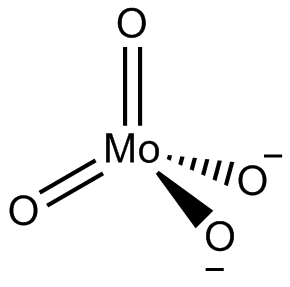
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.
Organotechnetium chemistry is the science of describing the physical properties, synthesis, and reactions of organotechnetium compounds, which are organometallic compounds containing carbon-to-technetium chemical bonds. The most common organotechnetium compounds are coordination complexes used as radiopharmaceutical imaging agents.

Technetium(IV) oxide, also known as technetium dioxide, is a chemical compound with the formula TcO2 which forms the dihydrate, TcO2·2H2O, which is also known as technetium(IV) hydroxide. It is a radioactive black solid which slowly oxidizes in air.
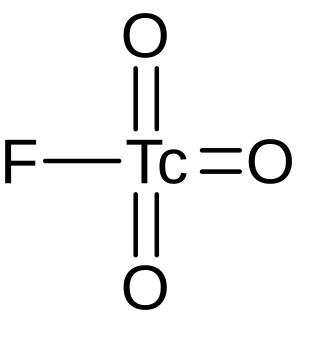
Pertechnetyl fluoride is an inorganic compound, a salt of technetium and hydrofluoric acid with the chemical formula TcO
3F. The compound was originally synthesized by H. Selig and G. Malm in 1963.
Technetium pentaluoride is a binary inorganic chemical compound of technetium metal and fluorine with the chemical formula TcF
5.

Sodium technetate(V) is an inorganic compound with the chemical formula NaTcO3. It is a perovskite material.
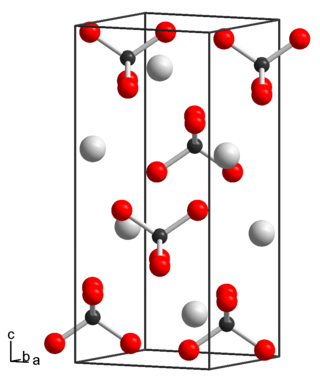
Rubidium pertechnetate is a pertechnetate of rubidium, with the chemical formula RbTcO4.
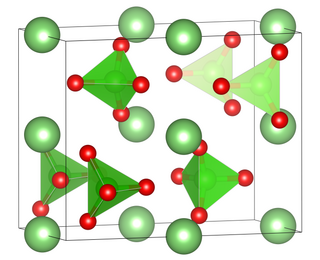
Caesium pertechnetate is the pertechnetate salt of caesium, with the chemical formula of CsTcO4.