Related Research Articles

Technetium is a chemical element; it has symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense of atomic number are both stable. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.
In chemistry, halogenation is a chemical reaction which introduces of one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

Radiopharmacology is radiochemistry applied to medicine and thus the pharmacology of radiopharmaceuticals. Radiopharmaceuticals are used in the field of nuclear medicine as radioactive tracers in medical imaging and in therapy for many diseases. Many radiopharmaceuticals use technetium-99m (Tc-99m) which has many useful properties as a gamma-emitting tracer nuclide. In the book Technetium a total of 31 different radiopharmaceuticals based on Tc-99m are listed for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood and tumors.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Tantalum(V) fluoride is the inorganic compound with the formula TaF5. It is one of the principal molecular compounds of tantalum. Characteristic of some other pentafluorides, the compound is volatile but exists as an oligomer in the solid state.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.

Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6 or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Rhenium pentafluoride is a binary inorganic compound of rhenium and fluorine with the chemical formula ReF5. This is a salt of rhenium and hydrofluoric acid.
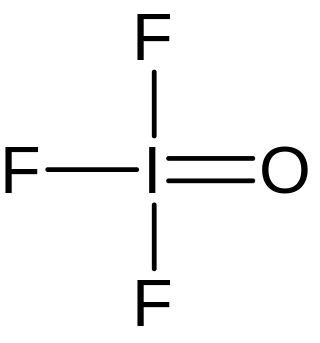
Iodosyl trifluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IOF3.
Iodosyl pentafluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IOF5.
Periodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO3F. The compound has been initially synthesized around 1950.
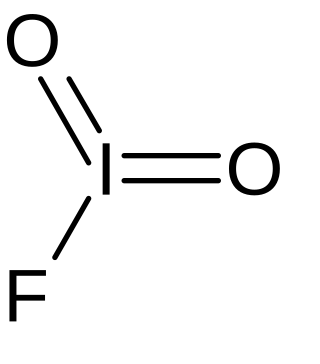
Iodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F. The compound was initially synthesized in 1951.
Iodine trifluoride dioxide is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F3. The compound was first obtained by Engelbrecht and Petersy in 1969.
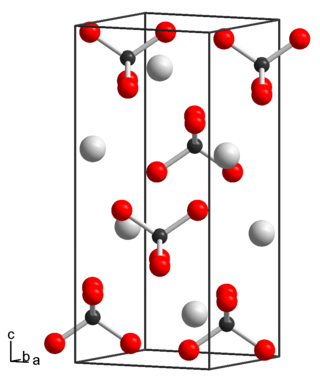
Rubidium pertechnetate is a pertechnetate of rubidium, with the chemical formula RbTcO4.
References
- ↑ "WebElements Periodic Table » Technetium » technetium pentafluoride". webelements.com. Retrieved 19 April 2023.
- ↑ Gutmann, Viktor (2 December 2012). Halogen Chemistry. Elsevier. p. 197. ISBN 978-0-323-14847-4 . Retrieved 19 April 2023.
- ↑ Schwochau, Klaus (21 November 2008). Technetium: Chemistry and Radiopharmaceutical Applications. John Wiley & Sons. p. 113. ISBN 978-3-527-61337-3 . Retrieved 19 April 2023.
- ↑ "Some physical properties of technetium pentafluoride". Journal of Inorganic and Nuclear Chemistry . 28: 231–232. 1 January 1976. doi:10.1016/0022-1902(76)80635-5. ISSN 0022-1902 . Retrieved 19 April 2023.
- ↑ Schwochau, Klaus (21 November 2008). Technetium: Chemistry and Radiopharmaceutical Applications. John Wiley & Sons. p. 114. ISBN 978-3-527-61337-3 . Retrieved 19 April 2023.
- ↑ Lide, David R. (29 June 2004). CRC Handbook of Chemistry and Physics, 85th Edition. CRC Press. p. 4-88. ISBN 978-0-8493-0485-9 . Retrieved 19 April 2023.
- ↑ Kemmitt, R. D. W.; Peacock, R. D. (26 January 2016). The Chemistry of Manganese, Technetium and Rhenium: Pergamon Texts in Inorganic Chemistry. Elsevier. p. 889. ISBN 978-1-4831-8762-4 . Retrieved 19 April 2023.