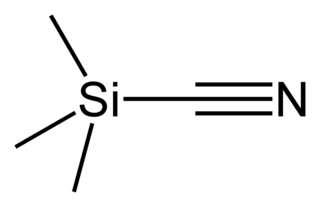Iron(III) chloride is the inorganic compound with the formula. Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.

Chromium(III) chloride (also called chromic chloride) describes any of several compounds with the formula CrCl3 · xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3 · 6 H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.
Chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
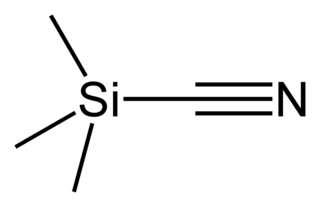
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.

Hexamethyldisiloxane (HMDSO) is an organosilicon compound with the formula O[Si(CH3)3]2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. The molecule is the protypical disiloxane and resembles a subunit of polydimethylsiloxane.
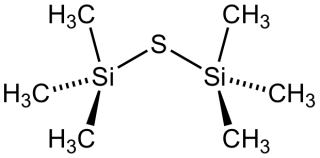
Bis(trimethylsilyl) sulfide is the chemical compound with the formula ((CH3)3Si)2S. Often abbreviated (tms)2S, this colourless, vile-smelling liquid is a useful aprotic source of "S2−" in chemical synthesis.

Trimethylsilyl azide ((CH3)3SiN3) is a chemical compound used as a reagent in organic chemistry.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si 3)2. It is commonly abbreviated as LiHMDS and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.
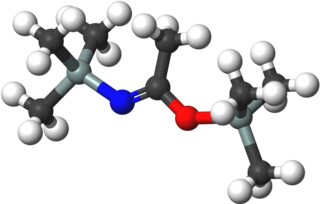
Bis(trimethylsilyl)acetamide (BSA) is an organosilicon compound with the formula Me3SiNC(OSiMe3)Me (Me = CH3). It is a colorless liquid that is soluble in diverse organic solvents, but reacts rapidly with compounds, including solvents and moisture, containing OH and NH functional groups. It is used in analytical chemistry for the derivatisation of compounds in analysis to increase their volatility, e.g. for gas chromatography. It is also used to introduce the trimethylsilyl protecting group in organic synthesis. A related reagent is N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA).
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. The process is the basis of organosilicon chemistry.

Bis(trimethylsilyl)acetylene (BTMSA) is an organosilicon compound with the formula Me3SiC≡CSiMe3 (Me = methyl). It is a colorless liquid that is soluble in organic solvents. This compound is used as a surrogate for acetylene.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.

Trimethylsilyl cyclopentadiene is an organosilicon compound with the chemical formula C5H5Si(CH3)3. It exists as a colorless liquid. It is used in the synthesis of some metal cyclopentadienyl complexes and has attracted interest for its fluxional structure.
Tris(trimethylsilyl)phosphine is the organophosphorus compound with the formula P(SiMe3)3 (Me = methyl). It is a colorless liquid that ignites in air and hydrolyses readily.

tert-Butyldimethylsilyl chloride is an organosilicon compound with the formula (Me3C)Me2SiCl (Me = CH3). It is commonly abbreviated as TBSCl or TBDMSCl. It is a silane containing two methyl groups, a tert-butyl group, and a reactive chloride. It is a colorless or white solid that is soluble in many organic solvents but reacts with water and alcohols. The compound is used to protect alcohols in organic synthesis. Examples can be found in the Nicolaou taxol total synthesis.

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer [LiCH2tms]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2tms)4(Et2O)2] and [Li2(μ-CH2tms)2(tmeda)2].