Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.

A dicarbonyl is a molecule containing two carbonyl (C=O) groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.

Acetylacetone is an organic compound with the chemical formula CH
3COCH
2COCH
3. It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH
3C(O)CH=(OH)CH
3. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion, a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds.
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an α,β-unsaturated carbonyl compound containing an electron withdrawing group. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C–C bonds. Many asymmetric variants exist.

Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula C
6H
8O
4. Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as [−O−(C
2)−O−(C=O)−(CH
2)−(C=O)−].

Cyclopentene is a chemical compound with the formula (CH2)3(CH)2. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes. Cyclopentene is produced industrially in large amounts by steam cracking of naphtha. It has few applications, and thus is mainly used as a component of gasoline.
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl halide.
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis.
The Paal–Knorr Synthesis in organic chemistry is a reaction that generates either furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes. Although the Paal–Knorr synthesis has seen widespread use, the mechanism wasn't fully understood until it was elucidated by V. Amarnath et al. in the 1990s.

1,2-Dioxin is a heterocyclic, organic, antiaromatic compound with the chemical formula C4H4O2. It is an isomeric form of 1,4-dioxin (or p-dioxin).
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.

Tetraphenylcyclopentadienone is an organic compound with the formula (C6H5)4C4CO. It is a dark purple to black crystalline solid that is soluble in organic solvents. It is an easily made building block for many organic and organometallic compounds.
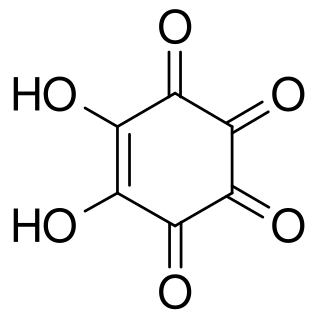
Rhodizonic acid is a chemical compound with formula C
6H
2O
6 or (CO)
4(COH)
2. It can be seen as a twofold enol and fourfold ketone of cyclohexene, more precisely 5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone.
Zoltan George Hajos is a Hungarian-born and trained American organic chemist. Originally an academic in his native Budapest, then an industrial chemist in the pharmaceutical industry, he is known for the Hajos–Parrish–Eder–Sauer–Wiechert reaction.

Dibenzoylmethane (DBM) is an organic compound with the formula (C6H5C(O))2CH2. DBM is the name for a 1,3-diketone, but the compound exists primarily as one of two equivalent enol tautomers. DBM (actually its enol) is a white solid. Due to their high photostability and UV-absorbing properties, derivatives of DBM such as avobenzone, have found applications as sunscreen products.

2-Hydroxy-3-methyl-2-cyclopenten-1-one is an organic compound related to 1,2-cyclopentanedione. It is the enol tautomer of the diketone 3-methylcyclopentane-1,2-dione. Being an enol, the compound is often called methylcyclopentenolone. It is a colorless solid.

1,3-Cyclohexanedione is an organic compound with the formula (CH2)4(CO)2. It is one of three isomeric cyclohexanediones. It is a colorless compound that occurs naturally. It is the substrate for cyclohexanedione hydrolase. The compound exists mainly as the enol tautomer.

Rick L. Danheiser is an American organic chemist and is the Arthur C. Cope Professor of Chemistry at the Massachusetts Institute of Technology and Chair of the MIT Faculty. His research involves the invention of new methods for the synthesis of complex organic compounds. Danheiser is known for the Danheiser annulation and Danheiser benzannulation reactions.

1,2-Cyclopentanedione is the organic compound with the formula (CH2)3(CO)2. It is one of two isomeric cyclopentanediones, the other being 1,3-cyclopentanedione. It was first prepared by base-induced condensation of di ethylglutarate with diethyloxalate, followed by hydrolysis of the resulting diketodiester followed by decarboxylation. The enol is predicted to be about 1-3 kcal/mol more stable than the diketo form. The enol structure has been confirmed by X-ray crystallography.












