Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (EC number 4.1.1).
Nepetalactone is a name for multiple iridoid analog stereoisomers. Nepetalactones are produced by Nepeta cataria (catnip) and many other plants belonging to the genus Nepeta, in which they protect these plants from herbivorous insects by functioning as insect repellents. They are also produced by many aphids, in which they are sex pheromones. Nepetalactones are cat attractants, and cause the behavioral effects that catnip induces in domestic cats. However, they affect visibly only about two thirds of adult cats. They produce similar behavioral effects in many other felids, especially in lions and jaguars. In 1941, the research group of Samuel M. McElvain was the first to determine the structures of nepetalactones and several related compounds.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
The aldol reaction is a reaction that combines two carbonyl compounds to form a new β-hydroxy carbonyl compound.

An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.

In organic chemistry, alkenols are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves deprotonation at the α position to the carbonyl group—i.e., removal of the hydrogen atom there as a proton H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate. The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.

Vinyl alcohol, also called ethenol or ethylenol, is the simplest enol. With the formula CH2CHOH, it is a labile compound that converts to acetaldehyde immediately upon isolation near room temperature. It is not a practical precursor to any compound.

Acetylacetone is an organic compound with the chemical formula CH3COCH2COCH3. It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH3C(O)CH=C(OH)CH3. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion, a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds.

Quinbolone, sold under the brand names Anabolicum and Anabolvis, is an androgen and anabolic steroid (AAS) which was previously marketed in Italy. It was developed by Parke-Davis as a viable orally administered AAS with little or no liver toxicity.

Fumarase is an enzyme that catalyzes the reversible hydration/dehydration of fumarate to malate. Fumarase comes in two forms: mitochondrial and cytosolic. The mitochondrial isoenzyme is involved in the Krebs cycle and the cytosolic isoenzyme is involved in the metabolism of amino acids and fumarate. Subcellular localization is established by the presence of a signal sequence on the amino terminus in the mitochondrial form, while subcellular localization in the cytosolic form is established by the absence of the signal sequence found in the mitochondrial variety.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.
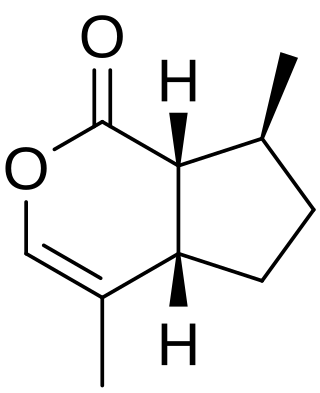
The Hajos–Parrish–Eder–Sauer–Wiechert reaction in organic chemistry is a proline catalysed asymmetric aldol reaction. The reaction is named after the principal investigators of the two groups who reported it simultaneously: Zoltan Hajos and David Parrish from Hoffmann-La Roche and Rudolf Wiechert and co-workers from Schering AG. Discovered in the 1970s the original Hajos-Parrish catalytic procedure – shown in the reaction equation, leading to the optically active bicyclic ketol – paved the way of asymmetric organocatalysis. The Eder-Sauer-Wiechert modification lead directly to the optically active enedione, through the loss of water from the bicyclic ketol shown in figure.

In organic chemistry, vinylogy is the transmission of electronic effects through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoaldehydes. Formylacetone, technically CH3(C=O)CH2CH=O, only exists in the ionized form CH3(C−O−)=CH−CH=O or CH3(C=O)−CH=CH−O−. Its adjectival form, vinylogous, is used to describe functional groups in which the standard moieties of the group are separated by a carbon–carbon double bond. For example, a compound with a carbon-carbon double bond between a carbonyl group and a hydroxyl group is referred to as a vinylogous carboxylic acid. The figure to the right shows two resonance forms of the anion.
In enzymology, phenylpyruvate tautomerase or Macrophage migration inhibitory factor is an enzyme that catalyzes the chemical reaction

In enzymology, a pyridoxine 5'-phosphate synthase (EC 2.6.99.2) is an enzyme that catalyzes the chemical reaction
The Saegusa–Ito oxidation is a chemical reaction used in organic chemistry. It was discovered in 1978 by Takeo Saegusa and Yoshihiko Ito as a method to introduce α-β unsaturation in carbonyl compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to yield the corresponding enone. The original publication noted its utility for regeneration of unsaturation following 1,4-addition with nucleophiles such as organocuprates.
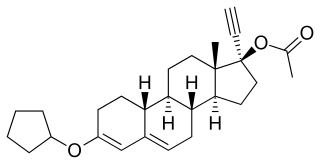
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

2-Hydroxy-3-methyl-2-cyclopenten-1-one is an organic compound related to 1,2-cyclopentanedione. It is the enol tautomer of the diketone 3-methylcyclopentane-1,2-dione. Being an enol, the compound is often called methylcyclopentenolone. It is a colorless solid.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed. It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity. In addition, it was able to maintain pregnancy in animals. Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive.














