
In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.

A dicarbonyl is a molecule containing two carbonyl (C=O) groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.
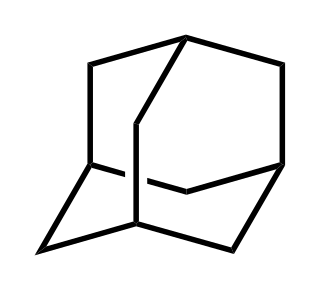
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.
Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.

Tautomers are structural isomers of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.
Sotolon is a lactone and an extremely powerful aroma compound, with the typical smell of fenugreek or curry at high concentrations and maple syrup, caramel, or burnt sugar at lower concentrations. Sotolon is the major aroma and flavor component of fenugreek seed and lovage, and is one of several aromatic and flavor components of artificial maple syrup. It is also present in molasses, aged rum, aged sake and white wine, flor sherry, roast tobacco, and dried fruiting bodies of the mushroom Lactarius helvus. Sotolon can pass through the body relatively unchanged, and consumption of foods high in sotolon, such as fenugreek, can impart a maple syrup aroma to one's sweat and urine. In some individuals with the genetic disorder maple syrup urine disease, it is spontaneously produced in their bodies and excreted in their urine, leading to the disease's characteristic smell.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.

Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula C6H8O4. Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as [−O−(C 2)−O−(C=O)−(CH2)−(C=O)−].
Menthone is a monoterpene with a minty flavor that occurs naturally in a number of essential oils. l-Menthone, shown at right, is the most abundant in nature of the four possible stereoisomers. It is structurally related to menthol, which has a secondary alcohol in place of the carbonyl. Menthone is used in flavoring, perfume and cosmetics for its characteristic aromatic and minty odor.

2-Pyridone is an organic compound with the formula C
5H
4NH(O). It is a colourless solid. It is well known to form hydrogen bonded dimers and it is also a classic case of a compound that exists as tautomers.

Pyrethrin I is one of the two pyrethrins, natural organic compounds with potent insecticidal activity. It is an ester of (+)-trans-chrysanthemic acid with (S)-(Z)-pyrethrolone.
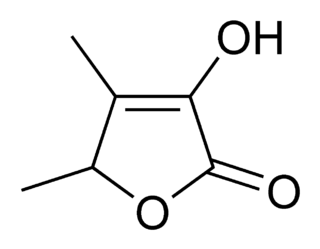
Triacetic acid lactone is an organic compound derived enzymatically from glucose. It is a light yellow solid that is soluble in organic solvents.
Decenoic acid is any mono-carboxylic acid with an unbranched chain of ten carbons connected by eight single bonds and one double bond; that is, a chemical compound with formula HO(O=)C–(CH
2)
k–CH=CH–(CH
2)
7-k–H, where k is between 0 and 7 inclusive.
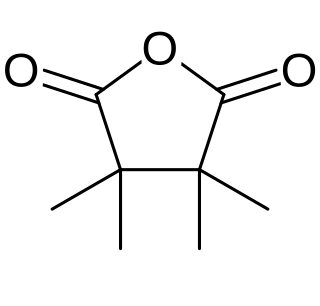
In chemistry, 3,3,4,4-tetramethyltetrahydrofuran-2,5-dione is a heterocyclic compound with the formula C
8H
12O
3, or (CH3)2(COC2COO)(CH3)2. It is a white crystalline solid with a pungent camphoraceous odor.
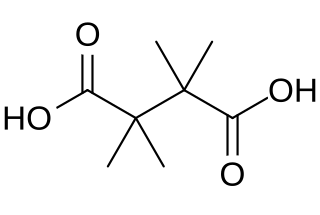
In chemistry, 2,2,3,3-tetramethylsuccinic acid or 2,2,3,3-tetramethylbutane-1,4-dioic acid is a dicarboxylic acid with the formula C
8H
14O
4, or HOOC-C(CH3)2-C(CH3)2-COOH.

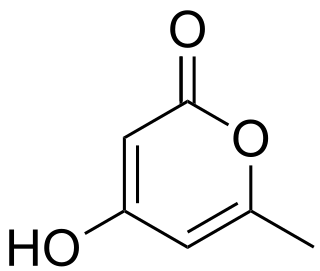
Tetronic acid is a chemical compound, classified as a γ-lactone, with the molecular formula C4H4O3.

1,3-Cyclohexanedione is an organic compound with the formula (CH2)4(CO)2. It is one of three isomeric cyclohexanediones. It is a colorless compound that occurs naturally. It is the substrate for cyclohexanedione hydrolase. The compound exists mainly as the enol tautomer.

1,2-Cyclopentanedione is the organic compound with the formula (CH2)3(CO)2. It is one of two isomeric cyclopentanediones, the other being 1,3-cyclopentanedione. It was first prepared by base-induced condensation of di ethylglutarate with diethyloxalate, followed by hydrolysis of the resulting diketodiester followed by decarboxylation. The enol is predicted to be about 1-3 kcal/mol more stable than the diketo form. The enol structure has been confirmed by X-ray crystallography.















