Honey is a sweet and viscous substance made by several species of bees, the best-known of which are honey bees. Honey is made and stored to nourish bee colonies. Bees produce honey by gathering and then refining the sugary secretions of plants or the secretions of other insects, like the honeydew of aphids. This refinement takes place both within individual bees, through regurgitation and enzymatic activity, and during storage in the hive, through water evaporation that concentrates the honey's sugars until it is thick and viscous.

Fructose, or fruit sugar, is a ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood.
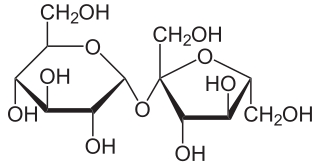
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula C
12H
22O
11.
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Furfural is only derived from dried biomass. In addition to ethanol, acetic acid, and sugar, furfural is one of the oldest organic chemicals available readily purified from natural precursors.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.

Molisch's test is a sensitive chemical test, named after Austrian botanist Hans Molisch, for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid or hydrochloric acid to produce an aldehyde, which condenses with two molecules of a phenol, resulting in a violet ring.
Humins are carbon-based macromolecular substances, that can be found in soil chemistry or as a by-product from saccharide-based biorefinery processes.

High-fructose corn syrup (HFCS), also known as glucose–fructose, isoglucose and glucose–fructose syrup, is a sweetener made from corn starch. As in the production of conventional corn syrup, the starch is broken down into glucose by enzymes. To make HFCS, the corn syrup is further processed by D-xylose isomerase to convert some of its glucose into fructose. HFCS was first marketed in the early 1970s by the Clinton Corn Processing Company, together with the Japanese Agency of Industrial Science and Technology, where the enzyme was discovered in 1965.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.

Furfuryl alcohol is an organic compound containing a furan substituted with a hydroxymethyl group. It is a colorless liquid, but aged samples appear amber. It possesses a faint odor of burning and a bitter taste. It is miscible with but unstable in water. It is soluble in common organic solvents.

Agave syrup, also known as maguey syrup or agave nectar, is a sweetener commercially produced from several species of agave, including Agave tequilana and Agave salmiana. Blue-agave syrup contains 56% fructose as a sugar providing sweetening properties.

2,5-Dimethylfuran is a heterocyclic compound with the formula (CH3)2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose.
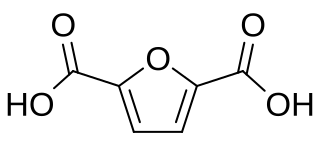
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.
István T. Horváth was a Hungarian American chemist, working on greener and more sustainable chemistry since its inception. In particular, he focuses on homogeneous transition metal catalysis and in situ spectroscopy. He was highly involved and very influential in the now enormous field of fluorous solvents and technologies.

2,5-Bis(hydroxymethyl)furan (BHMF) is a heterocyclic organic compound, and is a derivative of a broader class of compounds known as furans. It is produced from cellulose and has received attention as a biofeedstock. It is a white solid, although commercial samples can appear yellowish or tan.

Methoxymethylfurfural is an organic compound derived from dehydration of sugars and subsequent etherification with methanol. This colorless liquid is soluble in a wide range of solvents including lower alcohols. The molecule is a derivative of furan, containing both aldehyde and ether (methoxymethyl) functional groups. MMF has been detected in the leaves and roots of Chilean Jaborosa magellanica (Solanaceae). It has a typical odor suggestive of maraschino cherries. MMF can be made from a wide range of carbohydrate containing feedstocks including sugar, starch and cellulose using a chemical catalytic process and is a potential "carbon-neutral" feedstock for fuels and chemicals.
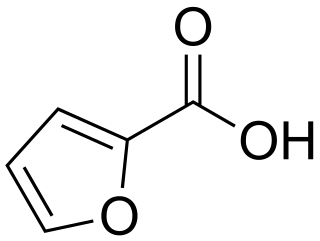
2-Furoic acid is an organic compound, consisting of a furan ring and a carboxylic acid side-group. Along with other furans, its name is derived from the Latin word furfur, meaning bran, from which these compounds were first produced. The salts and esters of furoic acids are known as furoates. 2-Furoic acid is most widely encountered in food products as a preservative and a flavouring agent, where it imparts a sweet, earthy flavour.
Dionisios G. Vlachos is an American chemical engineer, the Allan & Myra Ferguson Endowed Chair Professor of Chemical Engineering at the University of Delaware and director of the Catalysis Center for Energy Innovation, a U.S. Department of Energy - Energy Frontiers Research Center. Throughout his career at University of Delaware and the University of Minnesota, he has advanced the study of catalysts and reaction engineering including catalytic applications in biomass utilization, alkane conversion and zeolites. He is a fellow of the American Association for the Advancement of Science and recipient of the Wilhelm Award of the American Institute of Chemical Engineers (2011).
1,2,6-Hexanetriol is a trivalent alcohol with two primary and one secondary hydroxy group. It is similar to glycerol in many respects and is used as a substitute for glycerol in many applications due to its more advantageous properties, such as higher thermal stability and lower hygroscopicity.

2,5-Furandicarboxaldehyde (FDC) is an organic compound with the molecular formula C4H2O(CHO)2. It consists of a furan ring with aldehyde groups on the 2 and 5 position. It is therefore classified as a dialdehyde.
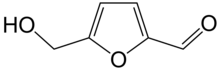


 [3]
[3]