| |||
| Names | |||
|---|---|---|---|
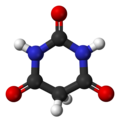
| Preferred IUPAC name 1,3-Diazinane-2,4,6-trione | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| 120502 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.598 | ||
| EC Number |
| ||
| 101571 | |||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H4N2O3 | |||
| Molar mass | 128.087 g·mol−1 | ||
| Appearance | White crystals | ||
| Melting point | 245 °C (473 °F; 518 K) | ||
| Boiling point | 260 °C (500 °F; 533 K) | ||
| 142 g/L (20 °C) | |||
| Acidity (pKa) | 4.01 (H2O) [1] | ||
| |||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
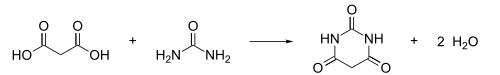
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active. The compound was first synthesised by Adolf von Baeyer.