In chemistry, a pentose is a monosaccharide with five carbon atoms. The chemical formula of all pentoses is C
5H
10O
5, and their molecular weight is 150.13 g/mol.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Furfural is only derived from lignocellulosic biomass, i.e., its origin is non-food or non-coal/oil based. Aside from ethanol, acetic acid and sugar it is one of the oldest renewable chemicals. It is also found in many processed foods and beverages.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.

Molisch's test is a sensitive chemical test, named after Austrian botanist Hans Molisch, for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid or hydrochloric acid to produce an aldehyde, which condenses with two molecules of a phenol, resulting in a violet ring.
Humins are carbon-based macromolecular substances, that can be found in soil chemistry or as a by-product from saccharide-based biorefinery processes.

Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.

Hydroxymethylfurfural (HMF), also 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars. It is a white low-melting solid which is highly soluble in both water and organic solvents. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups.

Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate.

Furfuryl alcohol is an organic compound containing a furan substituted with a hydroxymethyl group. It is a colorless liquid, but aged samples appear amber. It possesses a faint odor of burning and a bitter taste. It is miscible with but unstable in water. It is soluble in common organic solvents.
Chloroalkyl ethers are a class of organic compounds with the general structure R-O-(CH2)n-Cl, characterized as an ether connected to a chloromethyl group via an alkane chain.

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates.

γ-Valerolactone (GVL) is an organic compound with the formula C5H8O2. This colourless liquid is one of the more common lactones. GVL is chiral but is usually used as the racemate. It is readily obtained from cellulosic biomass and is a potential fuel and green solvent.
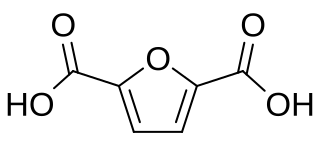
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.

2,5-Bis(hydroxymethyl)furan (BHMF) is a heterocyclic organic compound, and is a derivative of a broader class of compounds known as furans. It is produced from cellulose and has received attention as a biofeedstock. It is a white solid, although commercial samples can appear yellowish or tan.
Methyl vinyl ether is an organic compound with the chemical formula CH3OCH=CH2. A colorless gas, it is the simplest enol ether. It is used as a synthetic building block, as is the related compound ethyl vinyl ether (a liquid at room temperature).

2,2-Di-2-furylpropane is a condensation product of furan and acetone. It is a relatively high boiling liquid and is a precursor to the rubber additive bis(tetrahydrofuryl)propane used in the manufacture of high vinyl content rubber for high performance tires.

Allyl glycidyl ether is an organic compound used in adhesives and sealants and as a monomer for polymerization reactions. It is formally the condensation product of allyl alcohol and glycidol via an ether linkage. Because it contains both an alkene and an epoxide group, either group can be reacted selectively to yield a product where the other functional group remains intact for future reactions.

Furan resin refers to polymers produced from various furan compounds, of which the most common starting materials are furfuryl alcohol and furfural. In the resin and in the cured polyfurfurol, the furan rings are not connected by conjugation. The resins are generally used as binders for sand castings. The furan monomer is typically converted to a free-flowing resin with mild acid catalysis. Curing is achieved using strong acid.
















