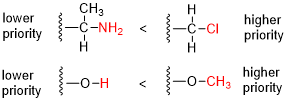In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of stereocenters will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4.

Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis-trans notation does not always correspond to E–Z isomerism, which is an absolute stereochemical description. In general, cis–trans stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is "superimposable" on its mirror image. Two objects can be superimposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.
In organic chemistry, dihydroxybenzenes (benzenediols) are organic compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three structural isomers: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.
In organic chemistry, hexene is a hydrocarbon with the chemical formula C6H12. The prefix "hex" is derived from the fact that there are 6 carbon atoms in the molecule, while the "-ene" suffix denotes that there is an alkene present—two carbon atoms are connected via a double bond. There are several isomers of hexene, depending on the position and geometry of the double bond in the chain. One of the most common industrially useful isomers is 1-hexene, an alpha-olefin. Hexene is used as a comonomer in the production of polyethylene.

2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is n-heptane). It is an important component of gasoline, frequently used in relatively large proportions (around 10%) to increase the knock resistance of fuel.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

m-Phenylenediamine, also called 1,3-diaminobenzene, is an organic compound with the formula C6H4(NH2)2. It is an isomer of o-phenylenediamine and p-phenylenediamine. This aromatic diamine is a colourless solid that appears as needles, but turns red or purple on exposure to air due to formation of oxidation products. Samples often come as colourless flakes and may darken in storage.

1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.

1-Fluoro-2,4-dinitrobenzene is a chemical that reacts with the N-terminal amino acid of polypeptides. This can be helpful for sequencing proteins.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
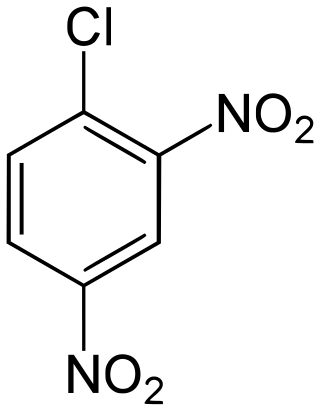
2,4-Dinitrochlorobenzene (DNCB) is an organic compound with the chemical formula (O2N)2C6H3Cl. It is a yellow solid that is soluble in organic solvents. It is an important intermediate for the industrial production of other compounds.
Zinin reaction or Zinin reduction was discovered by a Russian organic chemist Nikolay Zinin. This reaction involves conversion of nitro aromatic compounds like nitrobenzene to amines by reduction with sodium sulfides. The reaction exhibits excellent selectivity for the nitro group and is useful in cases where other easily reduced functional groups are present in the molecule. Moreover, dinitrobenzenes can often be reduced selectively to the nitroaniline.
Dinitrobenzenes are chemical compounds composed of a benzene ring and two nitro group (-NO2) substituents. The three possible arrangements of the nitro groups afford three isomers, 1,2-dinitrobenzene, 1,3-dinitrobenzene, and 1,4-dinitrobenzene. Each isomer has the chemical formula C6H4N2O4 and a molar mass of about 168.11 g/mol. 1,3-Dinitrobenzene is the most common isomer and it is used in the manufacture of explosives.
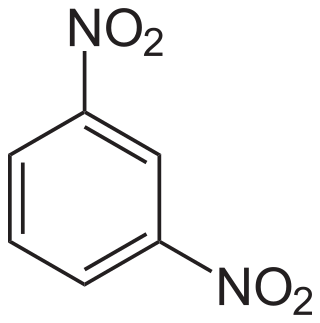
1,3-Dinitrobenzene is an organic compound with the formula C6H4(NO2)2. It is one of three isomers of dinitrobenzene. The compound is a yellow solid that is soluble in organic solvents.

Dinitroanilines are a class of chemical compounds with the chemical formula C6H5N3O4. They are derived from both aniline and dinitrobenzenes. There are six isomers: 2,3-dinitroaniline, 2,4-dinitroaniline, 2,5-dinitroaniline, 2,6-dinitroaniline, 3,4-dinitroaniline, and 3,5-dinitroaniline.
1,2-Dinitrobenzene is an organic compound with the formula C6H4(NO2)2. It is one of three isomers of dinitrobenzene. The compound is a white or colorless solid that is soluble in organic solvents. It is prepared from 2-nitroaniline by diazotization and treatment with sodium nitrite in the presence of a copper catalyst.
Ortho effect refers mainly to the set of steric effects and some bonding interactions along with polar effects caused by the various substituents which are in a given molecule, resulting in changes in its chemical and physical properties. In a general sense, the ortho effect is associated with substituted benzene compounds.
In chemistry, the Halex process is used to convert aromatic chlorides to the corresponding aromatic fluorides. The process entails Halide exchange, hence the name. The reaction conditions call for hot solution of the aryl chloride and anhydrous potassium fluoride. Typical solvents are dimethylsulfoxide, dimethylformamide, and sulfolane. Potassium chloride is generated in the process. The reaction is mainly applied to nitro-substituted aryl chlorides.