The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:

PyBOP is a reagent used to prepare amides from carboxylic acids and amines in the context of peptide synthesis. It can be prepared from 1-hydroxybenzotriazole and a chlorophosphonium reagent under basic conditions. It is a substitute for the BOP reagent that avoids the formation of the carcinogenic waste product HMPA. Thermal hazard analysis by differential scanning calorimetry (DSC) shows PyBOP is potentially explosive.
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972.

Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule. Methods to conjugate biomolecules are applied in various field, including medicine, diagnostics, biocatalysis and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme function, determining protein biodistribution, imaging specific biomarkers, and delivering drugs to targeted cells.

N-Hydroxysuccinimide (NHS) is an organic compound with the formula (CH2CO)2NOH. It is a white solid that is used as a reagent for preparing active esters in peptide synthesis. It can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride.
The molecular formula C5H4N4O (molar mass: 136.11 g/mol, exact mass: 136.0385 u) may refer to:

BOP (benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate) is a reagent commonly used for the synthesis of amides from carboxylic acids and amines in peptide synthesis. It can be prepared from 1-hydroxybenzotriazole and a chlorophosphonium reagent under basic conditions. This reagent has advantages in peptide synthesis since it avoids side reactions like the dehydration of asparagine or glutamine redisues. BOP has used for the synthesis of esters from the carboxylic acids and alcohols. BOP has also been used in the reduction of carboxylic acids to primary alcohols with sodium borohydride (NaBH4). Its use raises safety concerns since the carcinogenic compound HMPA is produced as a stoichiometric by-product.

HATU is a reagent used in peptide coupling chemistry to generate an active ester from a carboxylic acid. HATU is used along with Hünig's base (N,N-diisopropylethylamine), or triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used.

1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide is a water-soluble carbodiimide usually handled as the hydrochloride.
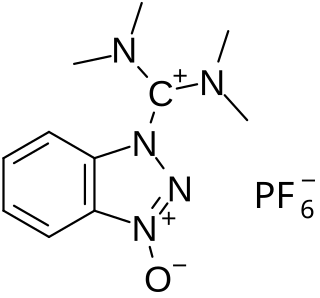
HBTU is a coupling reagent used in solid phase peptide synthesis. It was introduced in 1978 and shows resistance against racemization. It is used because of its mild activating properties.

PyAOP is a reagent used to prepare amides from carboxylic acids and amines in the context of peptide synthesis. It can be prepared from 1-hydroxy-7-azabenzotriazole (HOAt) and a chlorophosphonium reagent under basic conditions. It is a derivative of the HOAt family of amide bond forming reagents. It is preferred over HATU, because it does not engage in side reactions with the N-terminus of the peptide. Compared to the HOBt-containing analog PyBOP, PyAOP is more reactive due to the additional nitrogen in the fused pyridine ring of the HOAt moiety. Thermal hazard analysis by differential scanning calorimetry (DSC) shows PyAOP is potentially explosive.
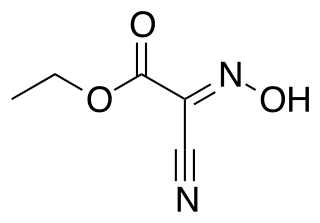
Ethyl cyanohydroxyiminoacetate (oxyma) is the oxime of ethyl cyanoacetate and finds use as an additive for carbodiimides, such as dicyclohexylcarbodiimide (DCC) in peptide synthesis. It acts as a neutralizing reagent for the basicity or nucleophilicity of the DCC due to its pronounced acidity and suppresses base catalyzed side reactions, in particular racemization.
HOAt is 1-Hydroxy-7-azabenzotriazole, a reagent used in organic chemistry.

HCTU is an amidinium coupling reagent used in peptide synthesis. It is analogous to HBTU. The HOBt moiety has a chlorine in the 6 position which improves reaction rates and the synthesis of difficult couplings HCTU and related reagents containing the 6-chloro-1-hydroxybenzotriazole moiety can be prepared by reaction with TCFH under basic conditions. It can exist in an N-form (guanidinium) or an O-form (uronium), but the N-form is generally considered to be more stable for this class of reagent. In vivo dermal sensitization studies according to OECD 429 confirmed HCTU is a strong skin sensitizer, showing a response at 0.50 wt% in the Local Lymph Node Assay (LLNA) placing it in Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Dermal Sensitization Category 1A.

1,1'-Thiocarbonyldiimidazole (TCDI) is a thiourea containing two imidazole rings. It is the sulfur analog of the peptide coupling reagent carbonyldiimidazole (CDI).
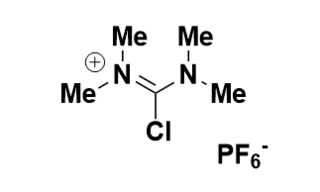
TCFH is an electrophilic amidine reagent used to activate a number of functional groups for reaction with nucleophilies. TCFH is most commonly used to activate carboxylic acids for reaction with amines in the context of amide bond formation and peptide synthesis.



















