| |||
 | |||
| Names | |||
|---|---|---|---|
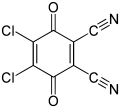
| Preferred IUPAC name 4,5-Dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile [2] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| Abbreviations | DDQ | ||
| ChemSpider | |||
| ECHA InfoCard | 100.001.402 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C8Cl2N2O2 | |||
| Molar mass | 227.00 g·mol−1 | ||
| Appearance | yellow to orange powder | ||
| Density | 1.7g/cm3 | ||
| Melting point | 210–215 °C (410–419 °F; 483–488 K) (decomposes) | ||
| Boiling point | 301.8 °C (575.2 °F; 575.0 K) at 760mmHg | ||
| reacts | |||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Danger | |||
| H301 | |||
| P264, P270, P301+P310, P321, P330, P405, P501 | |||
| Flash point | 136.3 °C (277.3 °F; 409.4 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, [3] phenols, [4] and steroid ketones. [5] DDQ decomposes in water, but is stable in aqueous mineral acid. [6]