
In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

N,N′-Dicyclohexylcarbodiimide (DCC or DCCD) is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water.
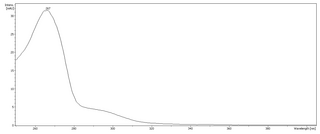
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed polyphenols.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. s-Triazine —the "symmetric" isomer—and its derivatives are useful in a variety of applications.

o-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. OPD is a white compound although samples appear darker owing to oxidation by air. It is isomeric with m-phenylenediamine and p-phenylenediamine.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.

Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor.

Picryl chloride is an organic compound with the formula ClC6H2(NO2)3. It is a bright yellow solid that is highly explosive, as is typical for polynitro aromatics such as picric acid. Its detonation velocity is 7,200 m/s.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.

1,3,5-Trinitrobenzene is one of three isomers of trinitrobenzene with the formula C6H3(NO2)3. A pale yellow solid, the compound is highly explosive.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.

Isovaleraldehyde organic compound, also known as 3-methylbutanal, with the formula (CH3)2CHCH2CHO. It is an aldehyde, a colorless liquid at STP, and found in low concentrations in many types of food. Commercially it is used as a reagent for the production of pharmaceuticals, perfumes and pesticides.

2,4,6-Tribromoaniline is a brominated derivative of aniline with the formula C6H4Br3N. It is used in organic synthesis of pharmaceuticals, agrochemicals and fire-extinguishing agents.

1,3,5-Tribromobenzene is an aryl bromide and isomer of tribromobenzene, also known as sym-tribromobenzene.
















