
PiHKAL: A Chemical Love Story is a book by Dr. Alexander Shulgin and Ann Shulgin, published in 1991. The subject of the work is psychoactive phenethylamine chemical derivatives, notably those that act as psychedelics and/or empathogen-entactogens. The main title, PiHKAL, is an acronym that stands for "Phenethylamines I Have Known and Loved."
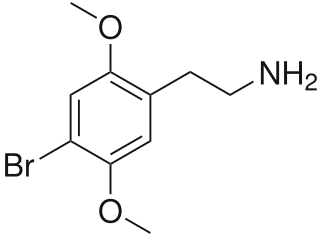
2C-B (4-bromo-2,5-dimethoxyphenethylamine) is a synthetic psychedelic drug of the 2C family, initially synthesized by Alexander Shulgin in 1974 and commonly used as a recreational drug. There is limited scientific information regarding the drug's pharmacokinetics and pharmacological effects in humans. The existing studies primarily classify 2C-B as a stimulant, and hallucinogen, and less commonly as an entactogen, and empathogen.

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.
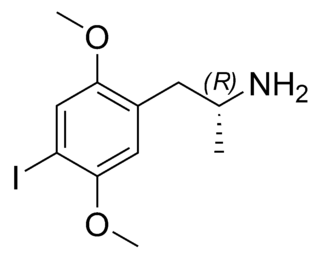
2,5-Dimethoxy-4-iodoamphetamine (DOI) is a psychedelic drug and a substituted amphetamine. Unlike many other substituted amphetamines, however, it is not primarily a stimulant. DOI has a stereocenter and R-(−)-DOI is the more active stereoisomer. In neuroscience research, [125I]-R-(−)-DOI is used as a radioligand and indicator of the presence of 5-HT2A serotonin receptors. DOI's effects have been compared to LSD, although there are differences that experienced users can distinguish. Besides the longer duration, the trip tends to be more energetic than an LSD trip, with more body load and a different subjective visual experience. The after effects include residual stimulation and difficulty sleeping, which, depending on the dose, may persist for days. While rare, it is sometimes sold as a substitute for LSD, or even sold falsely as LSD, which may be dangerous because DOI does not have the same established safety profile as LSD.
The Fischer oxazole synthesis is a chemical synthesis of an oxazole from a cyanohydrin and an aldehyde in the presence of anhydrous hydrochloric acid. This method was discovered by Emil Fischer in 1896. The cyanohydrin itself is derived from a separate aldehyde. The reactants of the oxazole synthesis itself, the cyanohydrin of an aldehyde and the other aldehyde itself, are usually present in equimolar amounts. Both reactants usually have an aromatic group, which appear at specific positions on the resulting heterocycle.
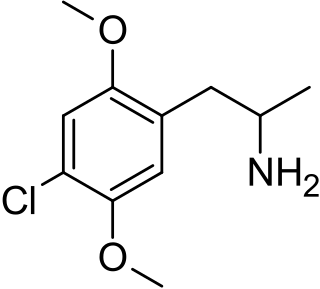
2,5-Dimethoxy-4-chloroamphetamine (DOC) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was presumably first synthesized by Alexander Shulgin, and was described in his book PiHKAL.

2C-TFM is a psychedelic phenethylamine of the 2C family. It was first synthesized in the laboratory of David E. Nichols. It has also been called 2C-CF3, a name derived from the Para-trifluoromethyl group it contains.
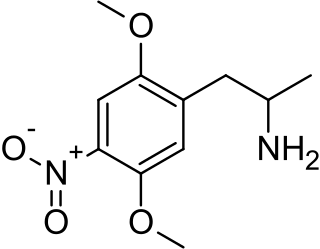
2,5-Dimethoxy-4-nitroamphetamine (DON) is a psychedelic drug and amphetamine. It is an analog of DOM and DOB. It is also closely related to 2C-N.

2,5-Dimethoxy-4-ethylamphetamine is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL.

Angels of Death is a 1986 compilation album by Hawkwind covering their RCA/Active Records period 1981–1982. It draws from the albums Sonic Attack (1981), Church of Hawkwind (1982) and Choose Your Masques (1982).
Ganesha (2,5-dimethoxy-3,4-dimethylamphetamine) is a lesser-known psychedelic drug. It is also a substituted amphetamine. It was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 24–32 mg. The drug is usually taken orally, although other routes such as rectally may also be used. Ganesha is synthesized from 2,5-dimethoxy-3,4-dimethylbenzaldehyde. Ganesha is the amphetamine analog of 2C-G. It is a particularly long lasting drug, with the duration listed in PiHKAL as being 18–24 hours, which might make it undesirable to some users. It is named after the Hindu deity, Ganesha. Very little is known about the dangers or toxicity of ganesha. Effects of ganesha include:

Beatrice is a lesser-known psychedelic drug. It is a substituted methamphetamine and a homolog of 2,5-dimethoxy-4-methylamphetamine (DOM). Beatrice was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 30 mg, and the duration listed as 6–10 hours. Beatrice produces a vague feeling of openness and receptiveness, and causes a stimulative effect. It also causes diarrhea. Very little data exists about the pharmacological properties, metabolism, and toxicity of beatrice.
Dimethoxyamphetamine (DMA) is a series of six lesser-known psychedelic drugs similar in structure to the three isomers of methoxyamphetamine and six isomers of trimethoxyamphetamine. The isomers are 2,3-DMA, 2,4-DMA, 2,5-DMA, 2,6-DMA, 3,4-DMA, and 3,5-DMA. Three of the isomers were characterized by Alexander Shulgin in his book PiHKAL. Little is known about their dangers or toxicity.

The Latvian Chess Championship is the annual national chess tournament of Latvia among men and women players, which was established in 1924. It is organized by the Latvian Chess Federation, previously - Latvian Chess Union.

2,5-Dimethoxy-4-trifluoromethylamphetamine (DOTFM) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized in 1994 by a team at Purdue University led by David E. Nichols. DOTFM is the alpha-methylated analogue of 2C-TFM, and is around twice as potent in animal studies. It acts as an agonist at the 5-HT2A and 5-HT2C receptors. In drug-substitution experiments in rats, DOTFM fully substituted for LSD and was slightly more potent than DOI.
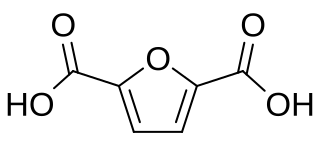
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.
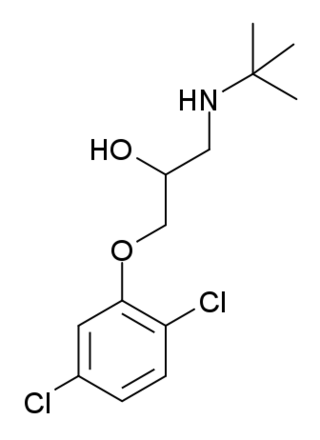
Cloranolol (Tobanum) is a beta blocker.
The molecular formula C6H8S (molar mass: 112.19 g/mol, exact mass: 112.0347 u) may refer to:

2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. Most compounds of this class are potent and long-lasting psychedelic drugs, and act as highly selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonists. A few bulkier derivatives such as DOAM have similarly high binding affinity for 5-HT2 receptors but instead act as antagonists, and so do not produce psychedelic effects though they retain amphetamine-like stimulant effects.

















