Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

Trinitrotoluene, more commonly known as TNT (and more specifically 2,4,6-trinitrotoluene), and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.
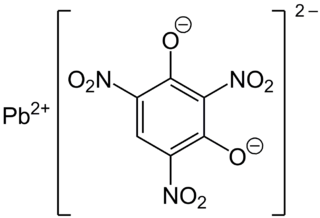
Lead styphnate (lead 2,4,6-trinitroresorcinate, C6HN3O8Pb ), whose name is derived from styphnic acid, is an explosive used as a component in primer and detonator mixtures for less sensitive secondary explosives. Lead styphnate is only slightly soluble in water and methanol. Samples of lead styphnate vary in color from yellow to gold, orange, reddish-brown, to brown. Lead styphnate is known in various polymorphs, hydrates, and basic salts. Normal lead styphnate monohydrate, monobasic lead styphnate, tribasic lead styphnate dihydrate, and pentabasic lead styphnate dehydrate as well as α, β polymorphs of lead styphnate exist.

In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.

A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compounds do undergo nucleophilic substitution. Just as normally nucleophilic alkenes can be made to undergo conjugate substitution if they carry electron-withdrawing substituents, so normally nucleophilic aromatic rings also become electrophilic if they have the right substituents.

TATB, triaminotrinitrobenzene or 2,4,6-triamino-1,3,5-trinitrobenzene is an aromatic explosive, based on the basic six-carbon benzene ring structure with three nitro functional groups (NO2) and three amine (NH2) groups attached, alternating around the ring.

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moisture, Na2S immediately hydrates to give sodium hydrosulfide.

1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its detonation velocity is 7,350 meters per second, which is comparable to TATB (triaminotrinitrobenzene).
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself.

Picryl chloride is an organic compound with the formula ClC6H2(NO2)3. It is a bright yellow solid that is highly explosive, as is typical for polynitro aromatics such as picric acid. Its detonation velocity is 7,200 m/s.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

2,4,6-Trimethylaniline is an organic compound with formula (CH3)3C6H2NH2. It is an aromatic amine that is of commercial interest as a precursor to dyes. It is prepared by selective nitration of mesitylene, avoiding oxidation of the methyl groups, followed by reduction of the resulting nitro group to the aniline.

1,3,5-Trinitrobenzene is one of three isomers of trinitrobenzene with the formula C6H3(NO2)3. A pale yellow solid, the compound is highly explosive.
Synthetic musks are a class of synthetic aroma compounds to emulate the scent of deer musk and other animal musks. Synthetic musks have a clean, smooth and sweet scent lacking the fecal notes of animal musks. They are used as flavorings and fixatives in cosmetics, detergents, perfumes and foods, supplying the base note of many perfume formulas. Most musk fragrance used in perfumery today is synthetic.

Trinitrobenzenesulfonic acid (C6H3N3O9S) is a nitroaryl oxidizing acid. Due to its extreme oxidative properties, if mixed with reducing agents including hydrides, sulfides, and nitrides, it may begin a vigorous reaction that culminates in almost immediate detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents because of the explosive tendencies of aromatic nitro compounds which increase in the presence of multiple nitro groups. Not much is known about this compound, but it is used as a peptide terminal amino group neutralizer and is currently being investigated for its effects on the immune system.
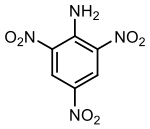
2,4-Dinitroaniline is a chemical compound with a formula of C6H5N3O4. It is used as an explosive and as a reagent to detect and characterize aldehydes and ketones.