A methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms — CH3. In formulas, the group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, it can be found on its own in any of three forms: anion, cation or radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.
Monosaccharides, also called simple sugars, are the simplest form of sugar and the most basic units (monomers) of carbohydrates. The general formula is C
nH
2nO
n, albeit not all molecules fitting this formula are carbohydrates. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are mirror images of each other that cannot appear identical simply by reorientation. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.

In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a relatively long time period as a stable rotational isomer or rotamer. When the time scale for interconversion is long enough for isolation of individual rotamers, the isomers are termed atropisomers. The ring-flip of substituted cyclohexanes constitutes another common form of conformational isomerism.
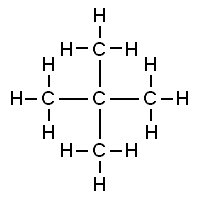
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.
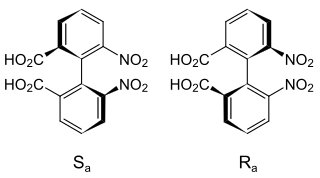
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.
The Curtin–Hammett principle is a principle in chemical kinetics proposed by David Yarrow Curtin and Louis Plack Hammett. It states that, for a reaction that has a pair of reactive intermediates or reactants that interconvert rapidly, each going irreversibly to a different product, the product ratio will depend both on the difference in energy between the two conformers and the energy barriers from each of the rapidly equilibrating isomers to their respective products. Stated another way, the product distribution reflects the difference in energy between the two rate-limiting transition states. As a result, the product distribution will not necessarily reflect the equilibrium distribution of the two intermediates. The Curtin–Hammett principle has been invoked to explain selectivity in a variety of stereo- and regioselective reactions. The relationship between the (apparent) rate constants and equilibrium constant is known as the Winstein-Holness equation.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic, in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

In stereochemistry, asymmetric induction describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.
In stereochemistry, topicity is the stereochemical relationship between substituents and the structure to which they are attached. Depending on the relationship, such groups can be heterotopic, homotopic, enantiotopic, or diastereotopic.

A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.
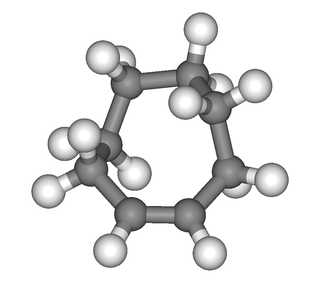
trans-Cyclooctene is a cyclic hydrocarbon with the formula [–(CH2)6CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor.

DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA.
Chiral Lewis acids (CLAs) are a type of Lewis acid catalyst that effects the chirality of the substrate as it reacts with it. In such reactions, the synthesis favors the formation of a specific enantiomer or diastereomer. The method then is an enantioselective asymmetric synthesis reaction. Since they affect chirality, they produce optically active products from optically inactive or mixed starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is formally known as an asymmetric induction. In this kind of Lewis acid. the electron-accepting atom is typically a metal, such as indium, zinc, lithium, aluminium, titanium, or boron. The chiral-altering ligands employed for synthesizing these acids most often have multiple Lewis basic sites that allow the formation of a ring structure involving the metal atom.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formulas — that is, same number of atoms of each element — but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.

In chemistry, inherent chirality is a property of asymmetry in molecules arising, not from a stereogenic or chiral center, but from a twisting of the molecule in 3-D space. The term was first coined by Volker Boehmer in a 1994 review, to describe the chirality of calixarenes arising from their non-planar structure in 3-D space.
Asymmetric ester hydrolysis with pig liver esterase is the enantioselective conversion of an ester to a carboxylic acid through the action of the enzyme pig liver esterase. Asymmetric ester hydrolysis involves the selective reaction of one of a pair of either enantiotopic or enantiomorphic ester groups.

Chirality is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek χειρ (kheir), "hand," a familiar chiral object.
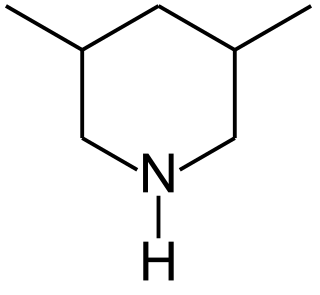
3,5-Dimethylpiperidines are chemical compounds with the formula C5H8(CH3)2NH. Two diastereomers exist: the achiral R,S isomer and the chiral R,R/S,S enantiomeric pair. 3,5-Dimethylpiperidine is a precursor to tibric acid.















