
In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed acetate esters or simply acetates. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound.

Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. Structurally, it is an ether with a methyl and phenyl group attached. Anisole is a standard reagent of both practical and pedagogical value.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demonstrated the reduction of ethyl oleate and n-butyl oleate to oleyl alcohol. Modified versions of which were subsequently refined and published in Organic Syntheses.

Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst. The metal is supported on activated carbon to maximize its surface area and activity.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Diglyme, or bis(2-methoxyethyl) ether, is an organic compound with the chemical formula (CH3OCH2CH2)2O. It is a colorless liquid with a slight ether-like odor. It is a solvent with a high boiling point. It is the dimethyl ether of diethylene glycol. The name diglyme is a portmanteau of diglycol methyl ether. It is miscible with water as well as organic solvents.

4-Bromoanisole is the organobromine compound with the formula CH3OC6H4Br. It is colorless liquid with a pleasant smell similar to that of anise seed. It is one of three isomers of bromoanisole, the others being 3-bromoanisole and 2-bromoanisole. It is the precursor to many 4-anisyl derivatives.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.

Trimethylsilylacetylene is the organosilicon compound with the formula (CH3)3SiC2H. A colorless liquid, "tms acetylene", as it is also called, is used as a source of "HC2−" in organic synthesis.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.
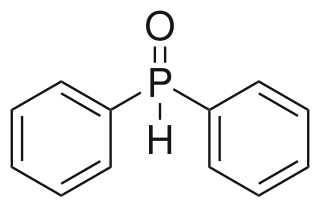
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents.

Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds, especially for vinylations.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.

Benzophenone imine is an organic compound with the formula of (C6H5)2C=NH. A pale yellow liquid, benzophenone imine is used as a reagent in organic synthesis.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.

Xylylene dibromide is an organic compound with the formula C6H4(CH2Br)2. It is an off-white solid that, like other benzyl halides, a strong lachrymator. It is a useful reagent owing to the convenient reactivity of the two C-Br bonds. Two other isomers are known, para- and meta-xylylene dibromide.














