
A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.
In chemistry, intramolecular describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.

In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (R−O−R’). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., −CH2CH2O−. Important members of this series are the tetramer (n = 4), the pentamer (n = 5), and the hexamer (n = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a cation, and a crown sitting on a person's head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are oxygen. Crown ethers are much broader than the oligomers of ethylene oxide; an important group are derived from catechol.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.

Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.

The Baker–Venkataraman rearrangement is the chemical reaction of 2-acetoxyacetophenones with base to form 1,3-diketones.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.
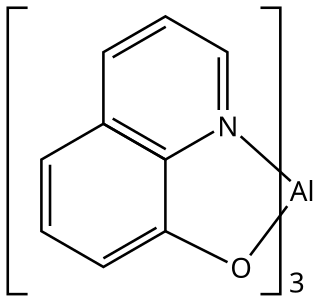
Tris(8-hydroxyquinolinato)aluminium is the chemical compound with the formula Al(C9H6NO)3. Widely abbreviated Alq3, it is a coordination complex wherein aluminium is bonded in a bidentate manner to the conjugate base of three 8-hydroxyquinoline ligands.
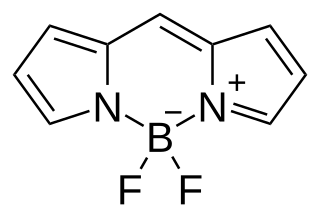
BODIPY is the technical common name of a chemical compound with formula C
9H
7BN
2F
2, whose molecule consists of a boron difluoride group BF
2 joined to a dipyrromethene group C
9H
7N
2; specifically, the compound 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene in the IUPAC nomenclature. The common name is an abbreviation for "boron-dipyrromethene". It is a red crystalline solid, stable at ambient temperature, soluble in methanol.
The Ferrier carbocyclization is an organic reaction that was first reported by the carbohydrate chemist Robert J. Ferrier in 1979. It is a metal-mediated rearrangement of enol ether pyrans to cyclohexanones. Typically, this reaction is catalyzed by mercury salts, specifically mercury(II) chloride.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.

Peter Michael Maitlis, FRS was a British organometallic chemist.

Trimethylsilyl iodide (iodotrimethylsilane or TMSI) is an organosilicon compound with the chemical formula (CH3)3SiI. It is a colorless, volatile liquid at room temperature.

Tris(2,2,2-trifluoroethyl) borate, also commonly referred to as the Sheppard amidation reagent, is a chemical compound with the formula B(OCH2CF3)3. This borate ester reagent is used in organic synthesis.

2,4,6-Tri-tert-butylphenol (2,4,6-TTBP) is a phenol symmetrically substituted with three tert-butyl groups and thus strongly sterically hindered. 2,4,6-TTBP is a readily oxidizable aromatic compound and a weak acid. It oxidizes to give the deep-blue 2,4,6-tri-tert-butylphenoxy radical. 2,4,6-TTBP is related to 2,6-di-tert-butylphenol, which is widely used as an antioxidant in industrial applications. These compounds are colorless solids.

Transition metal isocyanide complexes are coordination compounds containing isocyanide ligands. Because isocyanides are relatively basic, but also good pi-acceptors, a wide range of complexes are known. Some isocyanide complexes are used in medical imaging.
















