
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In polymer chemistry and materials science, a resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses mainly on naturally occurring resins.

Otto Wallach was a German chemist and recipient of the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.
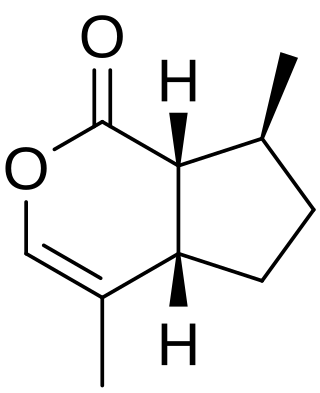
Nepetalactone is a name for multiple iridoid analog stereoisomers. Nepetalactones are produced by Nepeta cataria (catnip) and many other plants belonging to the genus Nepeta, in which they protect these plants from herbivorous insects by functioning as insect repellents. They are also produced by many aphids, in which they are sex pheromones. Nepetalactones are cat attractants, and cause the behavioral effects that catnip induces in domestic cats. However, they affect visibly only about two thirds of adult cats. They produce similar behavioral effects in many other felids, especially in lions and jaguars. In 1941, the research group of Samuel M. McElvain was the first to determine the structures of nepetalactones and several related compounds.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Together with geraniol, nerol, citronellol, linalool is one of the rose alcohols. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent.

Decalin, a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.

Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. An less-common isomeric form, having one of the three alkene units in a different position, is α-myrcene.
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide.
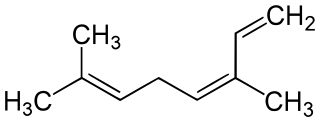
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is cis-3,7-dimethyl-1,3,7-octatriene. β-Ocimene is trans-3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, cis and trans, with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air. Like other terpenes, the ocimenes are nearly insoluble in water, but soluble in common organic solvents.
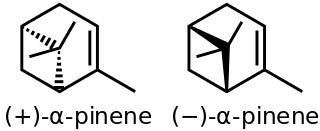
α-Pinene is an organic compound of the terpene class. It is one of the two isomers of pinene, the other being β-pinene. An alkene, it contains a reactive four-membered ring. It is found in the oils of many species of coniferous trees, notably Pinus and Picea species. It is also found in the essential oil of rosemary and Satureja myrtifolia. Both enantiomers are known in nature; (1S,5S)- or (−)-α-pinene is more common in European pines, whereas the (1R,5R)- or (+)-α-isomer is more common in North America. The enantiomers' racemic mixture is present in some oils such as eucalyptus oil and orange peel oil.

Cyclododecatrienes are cyclic trienes with the formula C12H18. Four isomers are known for 1,5,9-cyclododecatriene. The trans,trans,cis-isomer is a precursor in the production of nylon-12.

Sage oils are essential oils that come in several varieties:

Cannabis flower essential oil, also known as hemp essential oil, is an essential oil obtained by steam distillation from the flowers, panicles, stem, and upper leaves of the hemp plant. Hemp essential oil is distinct from hemp seed oil and hash oil: the former is a vegetable oil that is cold-pressed from the seeds of low-THC varieties of hemp, the latter is a THC-rich extract of dried female hemp flowers (marijuana) or resin (hashish).
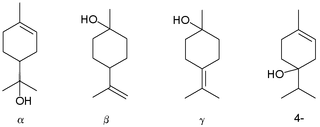
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil. Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. β- and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent.
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.

Sobrerol is a mucolytic.
Verbenol (2-pine-4-ol) is a group of stereoisomeric bicyclic monoterpene alcohols. These compounds have been found to be active components of insect pheromones and essential oils.

Pinane describes a pair of isomeric hydrocarbons. The isomers, actually diastereomers, are both chiral. They are the cis and trans isomers arising from the hydrogenation of the terpenes pinene. Both isomers undergo reaction with air (autoxidation) to give 2-pinane hydroperoxides. Partial reduction of these isomers gives 2-pinanol.



















