In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In enzymology, a (R)-2-hydroxyacid dehydrogenase (EC 1.1.1.272) is an enzyme that catalyzes the chemical reaction
In enzymology, a hydroxyacid-oxoacid transhydrogenase is an enzyme that catalyzes the chemical reaction

In enzymology, an (S)-2-hydroxy-acid oxidase (EC 1.1.3.15) is an enzyme that catalyzes the chemical reaction
The enzyme L-2-amino-4-chloropent-4-enoate dehydrochlorinase (EC 4.5.1.4) catalyzes the reaction
In enzymology, a 4-chlorobenzoate dehalogenase (EC 3.8.1.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-chlorobenzoyl-CoA dehalogenase (EC 3.8.1.7) is an enzyme that catalyzes the chemical reaction
In enzymology, an alkylhalidase (EC 3.8.1.1) is an enzyme that catalyzes the chemical reaction
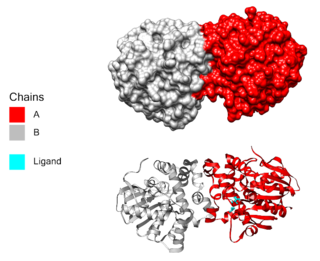
In enzymology, a haloacetate dehalogenase (EC 3.8.1.3) is an enzyme that catalyzes the chemical reaction
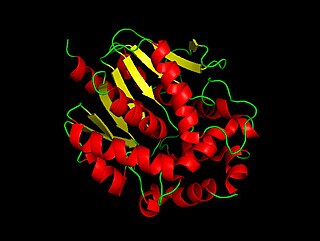
In enzymology, a haloalkane dehalogenase (EC 3.8.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-2-haloacid dehalogenase(EC 3.8.1.9), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-2-haloacid dehalogenase (EC 3.8.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxyglutarate synthase (EC 2.3.3.11) is an enzyme that catalyzes the chemical reaction
EC 3.8.1 is the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology's (NC-IUBMB) classification for C-Halide compounds.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
Pentachlorophenol monooxygenase (EC 1.14.13.50, pentachlorophenol dechlorinase, pentachlorophenol dehalogenase, pentachlorophenol 4-monooxygenase, PCP hydroxylase, pentachlorophenol hydroxylase, PcpB, PCB 4-monooxygenase, PCB4MO) is an enzyme with systematic name pentachlorophenol,NADPH:oxygen oxidoreductase (hydroxylating, dechlorinating). This enzyme catalyses the following chemical reaction
Phosphoethanolamine/phosphocholine phosphatase (EC 3.1.3.75, PHOSPHO1, 3X11A; systematic name phosphoethanolamine phosphohydrolase) is an enzyme highly expressed in mineralizing cells. This enzyme is implicated in bone and cartilage formation and catalyses the following chemical reactions:
2-haloacid dehalogenase (configuration-inverting) (EC 3.8.1.10, 2-haloalkanoic acid dehalogenase, 2-haloalkanoid acid halidohydrolase, DL-2-haloacid dehalogenase, DL-2-haloacid dehalogenase (inversion of configuration), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme with systematic name (S)-2-haloacid dehalogenase (configuration-inverting). This enzyme catalyses the following chemical reaction
Adsorbable Organic Halides (AOX) is a measure of the organic halogen load at a sampling site such as soil from a land fill, water, or sewage waste. The procedure measures chlorine, bromine, and iodine as equivalent halogens, but does not measure fluorine levels in the sample.

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.





