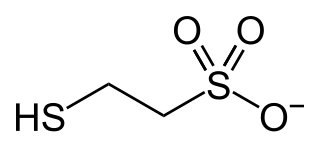
Coenzyme M is a coenzyme required for methyl-transfer reactions in the metabolism of archaeal methanogens, and in the metabolism of other substrates in bacteria. It is also a necessary cofactor in the metabolic pathway of alkene-oxidizing bacteria. CoM helps eliminate the toxic epoxides formed from the oxidation of alkenes such as propylene. The structure of this coenzyme was discovered by CD Taylor and RS Wolfe in 1974 while they were studying methanogenesis, the process by which carbon dioxide is transformed into methane in some anaerobic bacteria. The coenzyme is an anion with the formula HSCH
2CH
2SO−
3. It is named 2-mercaptoethanesulfonate and abbreviated HS–CoM. The cation is unimportant, but the sodium salt is most available. Mercaptoethanesulfonate contains both a thiol, which is the main site of reactivity, and a sulfonate group, which confers solubility in aqueous media.
In enzymology, a 2-(R)-hydroxypropyl-CoM dehydrogenase (EC 1.1.1.268) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-(S)-hydroxypropyl-CoM dehydrogenase (EC 1.1.1.269) is an enzyme that catalyzes the chemical reaction
In enzymology, a tetrahydromethanopterin S-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an alkene monooxygenase (EC 1.14.13.69) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-oxopropyl-CoM reductase (carboxylating) (EC 1.8.1.5) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-oxoacid CoA-transferase is an enzyme that catalyzes the chemical reaction
In enzymology, an acetate CoA-transferase is an enzyme that catalyzes the chemical reaction
The enzyme propanediol dehydratase (EC 4.2.1.28) catalyzes the chemical reaction

In enzymology, a homocitrate synthase (EC 2.3.3.14) is an enzyme that catalyzes the chemical reaction

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.
In enzymology, a dephospho-[reductase kinase] kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an ethanolaminephosphotransferase is an enzyme that catalyzes the chemical reaction

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
Phenylacetyl-CoA 1,2-epoxidase (EC 1.14.13.149, ring 1,2-phenylacetyl-CoA epoxidase, phenylacetyl-CoA monooxygenase, PaaAC, PaaABC(D)E) is an enzyme with systematic name phenylacetyl-CoA:oxygen oxidoreductase (1,2-epoxidizing). This enzyme catalyses the following chemical reaction
Propionate kinase is an enzyme with systematic name ATP:propanoate phosphotransferase. This enzyme catalyses the following chemical reaction
Methanogen homoaconitase (EC 4.2.1.114, methanogen HACN) is an enzyme with systematic name (R)-2-hydroxybutane-1,2,4-tricarboxylate hydro-lyase ((1R,2S)-1-hydroxybutane-1,2,4-tricarboxylate-forming). This enzyme catalyses the following chemical reaction
3-hydroxypropionyl-CoA dehydratase (EC 4.2.1.116) is an enzyme with systematic name 3-hydroxypropionyl-CoA hydro-lyase. This enzyme catalyses the following chemical reaction
In enzymology, a ceramide phosphoethanolamine synthase is an enzyme that catalyzes the chemical reaction
Coenzyme A transferases (CoA-transferases) are transferase enzymes that catalyze the transfer of a coenzyme A group from an acyl-CoA donor to a carboxylic acid acceptor. Among other roles, they are responsible for transfer of CoA groups during fermentation and metabolism of ketone bodies. These enzymes are found in all three domains of life.






