
Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.

Glyphosate is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by inhibiting the plant enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSP). It is used to kill weeds, especially annual broadleaf weeds and grasses that compete with crops. Its herbicidal effectiveness was discovered by Monsanto chemist John E. Franz in 1970. Monsanto brought it to market for agricultural use in 1974 under the trade name Roundup. Monsanto's last commercially relevant United States patent expired in 2000.

3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.

Paraquat (trivial name; ), or N,N′-dimethyl-4,4′-bipyridinium dichloride (systematic name), also known as methyl viologen, is an organic compound with the chemical formula [(C6H7N)2]Cl2. It is classified as a viologen, a family of redox-active heterocycles of similar structure. This salt is one of the most widely used herbicides. It is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic (lethal) to human beings and animals due to its redox activity, which produces superoxide anions. It has been linked to the development of Parkinson's disease and is banned in 58 countries.

MCPA is a widely used phenoxy herbicide introduced in 1945. It selectively controls broad-leaf weeds in pasture and cereal crops. The mode of action of MCPA is as an auxin, which are growth hormones that naturally exist in plants.

DCMU is an algicide and herbicide of the arylurea class that inhibits photosynthesis. It was introduced by Bayer in 1954 under the trade name of Diuron.

2,5-Dimethoxy-3,4-methylenedioxyamphetamine is a lesser-known psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin and was described in his book PiHKAL. Shulgin listed the dosage as 30–75 mg and the duration as 6–8 hours. He reported DMMDA as producing LSD-like images, mydriasis, ataxia, and time dilation. DMMDA isn't mentioned much in literature outside PiHKAL unlike 2C-B.

Triclocarban is an antibacterial chemical once common in, but now phased out of, personal care products like soaps and lotions. It was originally developed for the medical field. Although the mode of action is unknown, TCC can be effective in fighting infections by targeting the growth of bacteria such as Staphylococcus aureus. Additional research seeks to understand its potential for causing antibacterial resistance and its effects on organismal and environmental health.

Asulam is a herbicide invented by May & Baker Ltd, internally called M&B9057, that is used in horticulture and agriculture to kill bracken and docks. It is also used as an antiviral agent. It is currently marketed, by United Phosphorus Ltd - UPL, as "Asulox" which contains 400 g/L of asulam sodium salt.
MPP+ (1-methyl-4-phenylpyridinium) is a positively charged organic molecule with the chemical formula C12H12N+. It is a monoaminergic neurotoxin that acts by interfering with oxidative phosphorylation in mitochondria by inhibiting complex I, leading to the depletion of ATP and eventual cell death.
In enzymology, a 3,4-dichloroaniline N-malonyltransferase is an enzyme that catalyzes the chemical reaction
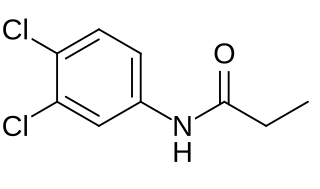
Propanil is a widely used contact herbicide. With an estimated use of about 8 million pounds in 2001, it is one of the more widely used herbicides in the United States. Propanil is said to be in use in approximately 400,000 acres of rice production each year.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
1,2-Dichloro-4-nitrobenzene is an organic compound with the formula 1,2-Cl2C6H3-4-NO2. This pale yellow solid is related to 1,2-dichlorobenzene by the replacement of one H atom with a nitro functional group. This compound is an intermediate in the synthesis of agrochemicals.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.
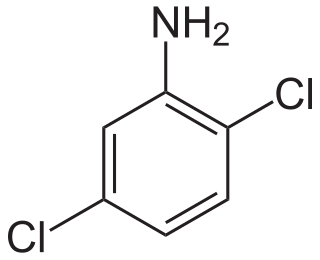
Dichloroanilines are chemical compounds which consist of an aniline ring substituted with two chlorine atoms and have the molecular formula C6H5Cl2N. There are six isomers, varying in the positions of the chlorine atoms around the ring relative to the amino group. As aniline derivatives, they are named with the amino group in position 1. They are all colorless, although commercial samples can appear colored due to the presence of impurities. Several derivatives are used in the production of dyes and herbicides.
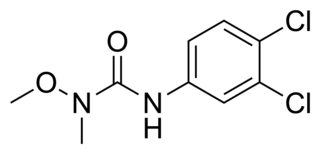
Linuron is a phenylurea herbicide that is used to control the growth of grass and weeds for the purpose of supporting the growth of crops like soybeans.

Methyldichlorophosphine (alternatively known as dichloro(methyl)phosphane, SW and methylphosphonous dichloride) is an organophosphorus compound with the chemical formula CH3PCl2. It is a colorless, corrosive, flammable, and highly reactive liquid with a pungent odor.

Cyanazine is a herbicide that belongs to the group of triazines. Cyanazine inhibits photosynthesis and is therefore used as a herbicide.
3,5-Dichloroaniline is an organic compound with the formula C6H3Cl2(NH2). It is one of several isomers of dichloroaniline. It is a colorless solid although commercial samples often appear colored. It is produced by hydrogenation of 3,5-dichloronitrobenzene. It is a precursor to the fungicide vinclozolin.





















