 | |
| Names | |
|---|---|
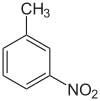
| Preferred IUPAC name 1-Methyl-3-nitrobenzene | |
| Other names m-Nitrotoluene | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.480 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C7H7NO2 | |
| Molar mass | 137.138 g·mol−1 |
| Appearance | yellow liquid [1] |
| Odor | mild, aromatic [1] |
| Density | 1.1581 g·cm−3 @ 20°C [2] |
| Melting point | 15.5 °C (59.9 °F; 288.6 K) [2] |
| Boiling point | 232 °C (450 °F; 505 K) [2] |
| 0.05% (20°C) [1] | |
| Vapor pressure | 0.1 mmHg (20°C) [1] |
| −72.71·10−6 cm3/mol | |
| Hazards | |
| Flash point | 106 °C; 223 °F; 379 K [1] |
| Explosive limits | 1.6%-? [1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 5 ppm (30 mg/m3) [skin] [1] |
REL (Recommended) | TWA 2 ppm (11 mg/m3) [skin] [1] |
IDLH (Immediate danger) | 200 ppm [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
3-Nitrotoluene or meta-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is one of three isomers of nitrotoluene. A yellow liquid, it is used in the manufacture of meta-toluidine, which is an intermediate in the production of various dyes. [3]