
A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

Catechol-O-methyltransferase is one of several enzymes that degrade catecholamines, catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-O-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957.
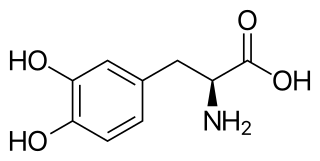
l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.
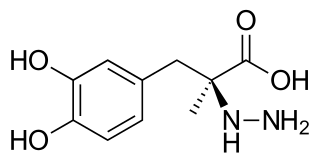
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of administered levodopa to cross the blood–brain barrier for central nervous system effect, instead of being peripherally metabolised into substances unable to cross said barrier.

Methyldopa, sold under the brand name Aldomet among others, is a medication used for high blood pressure. It is one of the preferred treatments for high blood pressure in pregnancy. For other types of high blood pressure including very high blood pressure resulting in symptoms other medications are typically preferred. It can be given by mouth or injection into a vein. Onset of effects is around 5 hours and they last about a day.

Benserazide is a peripherally acting aromatic L-amino acid decarboxylase or DOPA decarboxylase inhibitor, which is unable to cross the blood–brain barrier.

Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate dopamine-related activity. For example, certain proteins such as the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and dopamine receptors can be classified as dopaminergic, and neurons that synthesize or contain dopamine and synapses with dopamine receptors in them may also be labeled as dopaminergic. Enzymes that regulate the biosynthesis or metabolism of dopamine such as aromatic L-amino acid decarboxylase or DOPA decarboxylase, monoamine oxidase (MAO), and catechol O-methyl transferase (COMT) may be referred to as dopaminergic as well. Also, any endogenous or exogenous chemical substance that acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons that synapse onto neurons that release dopamine or express dopamine receptors) can also be said to have dopaminergic effects, two prominent examples being opioids, which enhance dopamine release indirectly in the reward pathways, and some substituted amphetamines, which enhance dopamine release directly by binding to and inhibiting VMAT2.

Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.
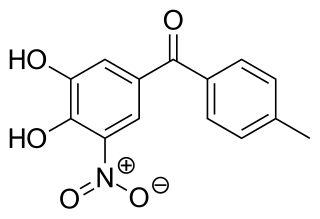
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.

A dopamine agonist(DA) is a compound that activates dopamine receptors. There are two families of dopamine receptors, D1-like and D2-like. They are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used in the treatment of Parkinson's disease, and to a lesser extent, in hyperprolactinemia and restless legs syndrome. They are also used off-label in the treatment of clinical depression. The use of dopamine agonists is associated with impulse control disorders and dopamine agonist withdrawal syndrome (DAWS).
Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

A catechol-O-methyltransferase(COMT) inhibitor is a drug that inhibits the enzyme catechol-O-methyltransferase. This enzyme methylates catecholamines such as dopamine, norepinephrine and epinephrine. It also methylates levodopa. COMT inhibitors are indicated for the treatment of Parkinson's disease in combination with levodopa and an aromatic L-amino acid decarboxylase inhibitor. The therapeutic benefit of using a COMT inhibitor is based on its ability to prevent the methylation of levodopa to 3-O-methyldopa, thus increasing the bioavailability of levodopa. COMT inhibitors significantly decrease off time in people with Parkinson's disease also taking carbidopa/levodopa.

Droxidopa is a synthetic amino acid precursor which acts as a prodrug to the neurotransmitter norepinephrine (noradrenaline). Unlike norepinephrine, droxidopa is capable of crossing the protective blood–brain barrier (BBB).
Carbidopa/levodopa/entacapone, sold under the brand name Stalevo among others, is a dopaminergic fixed-dose combination medication that contains carbidopa, levodopa, and entacapone for the treatment of Parkinson's disease.

An aromatic L-amino acid decarboxylase inhibitor is a medication of type enzyme inhibitor which inhibits the synthesis of dopamine by the enzyme aromatic L-amino acid decarboxylase. It is used to inhibit the decarboxylation of L-DOPA to dopamine outside the brain, i.e. in the blood. This is primarily co-administered with L-DOPA to combat Parkinson's disease. Administration can prevent common side-effects, such as nausea and vomiting, as a result of interaction with D2 receptors in the vomiting center located outside the blood–brain barrier.
Gene therapy in Parkinson's disease consists of the creation of new cells that produce a specific neurotransmitter (dopamine), protect the neural system, or the modification of genes that are related to the disease. Then these cells are transplanted to a patient with the disease. There are different kinds of treatments that focus on reducing the symptoms of the disease but currently there is no cure.
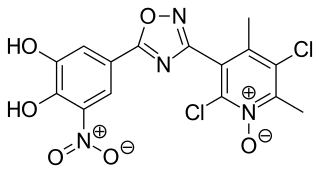
Opicapone, sold under the brand name Ongentys, is a medication which is administered together with levodopa in people with Parkinson's disease. Opicapone is a catechol-O-methyltransferase (COMT) inhibitor.
Aromatic L-amino acid decarboxylase deficiency, also known as AADC deficiency, is a rare genetic disorder caused by mutations in the DDC gene, which encodes an enzyme called aromatic L-amino acid decarboxylase.

Monoamine precursors are precursors of monoamines and monoamine neurotransmitters in the body. The amino acids L-tryptophan and L-5-hydroxytryptophan are precursors of serotonin and melatonin, while the amino acids L-phenylalanine, L-tyrosine, and L-DOPA (levodopa) are precursors of dopamine, epinephrine (adrenaline), and norepinephrine (noradrenaline). Administration of monoamine precursors can increase the levels of monoamine neurotransmitters in the body and brain. Monoamine precursors may be used in combination with peripherally selective aromatic L-amino acid decarboxylase inhibitors such as carbidopa and benserazide. Carbidopa/levodopa is used to increase brain dopamine levels in the treatment of Parkinson's disease while carbidopa/oxitriptan (EVX-101) is under development as an antidepressant for possible use in the treatment of depression.






















