
A liquid-crystal display (LCD) is a flat-panel display or other electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers to display information. Liquid crystals do not emit light directly but instead use a backlight or reflector to produce images in color or monochrome.
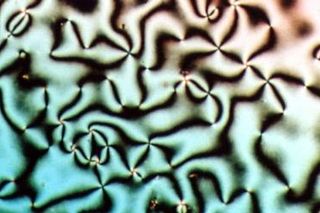
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties. The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates and Fermionic condensates, neutron-degenerate matter, and quark–gluon plasma.
A mesogen is a compound that displays liquid crystal properties. Mesogens can be described as disordered solids or ordered liquids because they arise from a unique state of matter that exhibits both solid- and liquid-like properties called the liquid crystalline state. This liquid crystalline state (LC) is called the mesophase and occurs between the crystalline solid (Cr) state and the isotropic liquid (Iso) state at distinct temperature ranges.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
A biaxial nematic is a spatially homogeneous liquid crystal with three distinct optical axes. This is to be contrasted to a simple nematic, which has a single preferred axis, around which the system is rotationally symmetric. The symmetry group of a biaxial nematic is i.e. that of a rectangular right parallelepiped, having 3 orthogonal axes and three orthogonal mirror planes. In a frame co-aligned with optical axes the second rank order parameter tensor, the so-called Q tensor of a biaxial nematic has the form

Biphenyl is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen may use the prefixes xenyl or diphenylyl.

The twisted nematic effect (TN-effect) was a major technological breakthrough that made the manufacture of large, thin liquid crystal displays practical and cost competitive. Unlike earlier flat-panel displays, TN-cells did not require a current to flow for operation and used low operating voltages suitable for use with batteries. The introduction of TN-effect displays led to their rapid expansion in the display field, quickly pushing out other common technologies like monolithic LEDs and CRTs for most electronics. By the 1990s, TN-effect LCDs were largely universal in portable electronics, although since then, many applications of LCDs adopted alternatives to the TN-effect such as in-plane switching (IPS) or vertical alignment (VA).

A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces, dipole–dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic interactions. Van der Waals, dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, and halogen bonding are typically much weaker than the forces holding together other solids: metallic, ionic, and network solids.

George William Gray was a Professor of Organic Chemistry at the University of Hull who was instrumental in developing the long-lasting materials which made liquid crystal displays possible. He created and systematically developed liquid crystal materials science, and established a method of practical molecular design. Gray was recipient of the 1995 Kyoto Prize in Advanced Technology.

Martin Schadt is a Swiss physicist and inventor.

Lyotropic liquid crystals result when amphiphiles, which are both hydrophobic and hydrophilic, dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water.

In chemistry and chemical physics, a mesophase or mesomorphic phase is a phase of matter intermediate between solid and liquid. Gelatin is a common example of a partially ordered structure in a mesophase. Further, biological structures such as the lipid bilayers of cell membranes are examples of mesophases. Mobile ions in mesophases are either orientationally or rotationally disordered while their centers are located at the ordered sites in the crystal structure. Mesophases with long-range positional order but no orientational order are plastic crystals, whereas those with long-range orientational order but only partial or no positional order are liquid crystals.
A blue phase mode LCD is a liquid crystal display (LCD) technology that uses highly twisted cholesteric phases in a blue phase. It was first proposed in 2007 to obtain a better display of moving images with, for example, frame rates of 100–120 Hz to improve the temporal response of LCDs. This operational mode for LCDs also does not require anisotropic alignment layers and thus theoretically simplifies the LCD manufacturing process.
There are various classifications of the electro-optical modes of liquid crystal displays (LCDs).

Sivaramakrishna Chandrasekhar FNA, FRS was an Indian physicist who won the Royal Medal in 1994. He was the founder-president of the International Liquid Crystal Society.
A liquid-crystal laser is a laser that uses a liquid crystal as the resonator cavity, allowing selection of emission wavelength and polarization from the active laser medium. The lasing medium is usually a dye doped into the liquid crystal. Liquid-crystal lasers are comparable in size to diode lasers, but provide the continuous wide spectrum tunability of dye lasers while maintaining a large coherence area. The tuning range is typically several tens of nanometers. Self-organization at micrometer scales reduces manufacturing complexity compared to using layered photonic metamaterials. Operation may be either in continuous wave mode or in pulsed mode.

Antal I. "Tony" Jákli is a Hungarian-American physicist and professor of chemical physics at Kent State University. He is known for his work with bent-core, flexoelectric, and ferroelectric liquid crystals.

Nelamangala Vedavyasachar Madhusudana is an Indian physicist and an emeritus scientist at Raman Research Institute. Known for his research on liquid crystals, Madhusudhana is an elected fellow of Indian Academy of Sciences and Indian National Science Academy. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, for his contributions to physical sciences in 1989.

Yuriy Reznikov was a Ukrainian physicist, Head of the Department of Crystals at NASU Institute of Physics and a world-renown expert in the field of liquid crystals. He is known for his work on photoalignment, "giant" optical non-linearity of liquid crystals and nano-colloids.


















