Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.
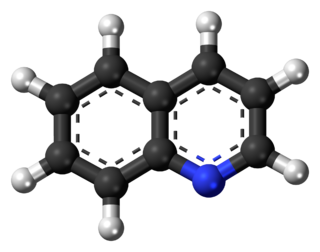
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers.

In organic chemistry, enols are a type of Functional group or intermediate in organic chemistry containing a group with the formula C=C(OH). The term enol is an abbreviation of alkenol, a portmanteau deriving from "-ene"/"alkene" and the "-ol". Many kinds of enols are known.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
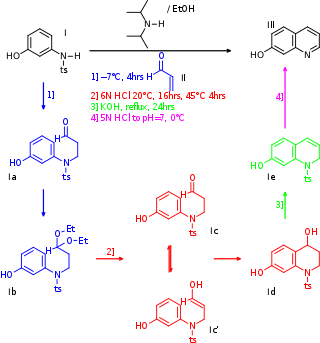
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction in which a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds can save time and resources while increasing chemical yield.

The Camps quinoline synthesis is a chemical reaction whereby an o-acylaminoacetophenone is transformed into two different hydroxyquinolines using hydroxide ion.
1,1′-Bi-2-naphthol (BINOL) is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation. The specific rotation of the two enantiomers is 35.5° (c = 1 in THF), with the R enantiomer being the dextrorotary one. BINOL is a precursor for another chiral ligand called BINAP. The volumetric mass density of the two enantiomers is 0.62 g cm−3.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen. Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation:
The Niementowski quinoline synthesis is the chemical reaction of anthranilic acids and ketones to form γ-hydroxyquinoline derivatives.

The Bartoli indole synthesis is the chemical reaction of ortho-substituted nitroarenes and nitrosoarenes with vinyl Grignard reagents to form substituted indoles.

In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insecticides and a number of quinolone antibiotics. However, the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.
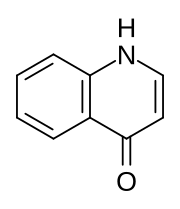
The Conrad–Limpach synthesis is the condensation of anilines (1) with β-ketoesters (2) to form 4-hydroxyquinolines (4) via a Schiff base (3). The overall reaction type is a combination of both an addition reaction as well as a rearrangement reaction. This reaction was discovered by Max Conrad (1848–1920) and Leonhard Limpach (1852–1933) in 1887 while they were studying the synthesis of quinoline derivatives.
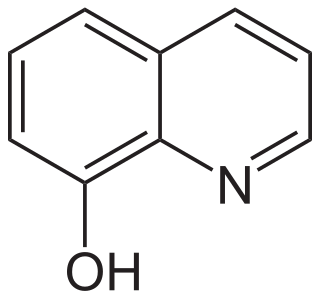
8-Hydroxyquinoline is an organic compound derived from the heterocycle quinoline. A colorless solid, its conjugate base is a chelating agent, which is used for the quantitative determination of metal ions.
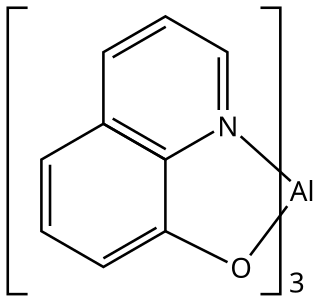
Tris(8-hydroxyquinolinato)aluminium is the chemical compound with the formula Al(C9H6NO)3. Widely abbreviated Alq3, it is a coordination complex wherein aluminium is bonded in a bidentate manner to the conjugate base of three 8-hydroxyquinoline ligands.

The Knorr quinoline synthesis is an intramolecular organic reaction converting a β-ketoanilide to a 2-hydroxyquinoline using sulfuric acid. This reaction was first described by Ludwig Knorr (1859–1921) in 1886
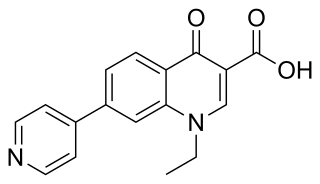
Rosoxacin is a quinolone antibiotic indicated for the treatment of urinary tract infections and certain sexually transmitted diseases. Rosoxacin is not available in the United States.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould–Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould–Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.