
γ-Aminobutyric acid, or GABA, is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.

Phenelzine, sold under the brand name Nardil, among others, is a non-selective and irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class which is primarily used as an antidepressant and anxiolytic. Along with tranylcypromine and isocarboxazid, phenelzine is one of the few non-selective and irreversible MAOIs still in widespread clinical use.

Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare autosomal recessive disorder of the degradation pathway of the inhibitory neurotransmitter γ-aminobutyric acid, or GABA. The disorder has been identified in approximately 350 families, with a significant proportion being consanguineous families. The first case was identified in 1981 and published in a Dutch clinical chemistry journal that highlighted a number of neurological conditions such as delayed intellectual, motor, speech, and language as the most common manifestations. Later cases reported in the early 1990s began to show that hypotonia, hyporeflexia, seizures, and a nonprogressive ataxia were frequent clinical features as well.

Succinic semialdehyde (SSA) is a GABA metabolite. It is formed from GABA by the action of GABA transaminase (4-aminobutyrate aminotransferase) and further oxidised to become succinic acid, which enters TCA cycle. SSA is oxidized into succinic acid by the enzyme succinic semialdehyde dehydrogenase, which uses NAD+ as a cofactor. When the oxidation of succinic semialdehyde to succinic acid is impaired, accumulation of succinic semialdehyde takes place which leads to succinic semialdehyde dehydrogenase deficiency.

In enzymology, 4-aminobutyrate transaminase, also called GABA transaminase or 4-aminobutyrate aminotransferase, or GABA-T, is an enzyme that catalyzes the chemical reaction:
In enzymology, an alanine-glyoxylate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, an aminolevulinate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a diaminobutyrate-pyruvate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a N6-acetyl-beta-lysine transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-3-amino-2-methylpropionate—pyruvate transaminase is an enzyme that catalyzes the chemical reaction

Succinate-semialdehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH5A1 gene.

Gabaculine is a naturally occurring neurotoxin first isolated from the bacteria Streptomyces toyacaensis, which acts as a potent and irreversible GABA transaminase inhibitor, and also a GABA reuptake inhibitor. Gabaculine is also known as 3-amino-2,3-dihydrobenzoic acid hydrochloride and 5-amino cyclohexa-1,3 dienyl carboxylic acid. Gabaculine increased GABA levels in the brain and had an effect on convulsivity in mice.
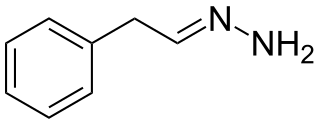
Phenylethylidenehydrazine (PEH), also known as 2-phenylethylhydrazone or β-phenylethylidenehydrazine, is an inhibitor of the enzyme GABA transaminase (GABA-T). It is a metabolite of the antidepressant phenelzine and is responsible for its elevation of GABA concentrations. PEH may contribute to phenelzine's anxiolytic effects.
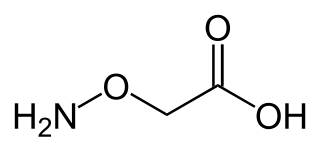
Aminooxyacetic acid, often abbreviated AOA or AOAA, is a compound that inhibits 4-aminobutyrate aminotransferase (GABA-T) activity in vitro and in vivo, leading to less gamma-aminobutyric acid (GABA) being broken down. Subsequently, the level of GABA is increased in tissues. At concentrations high enough to fully inhibit 4-aminobutyrate aminotransferase activity, aminooxyacetic acid is indicated as a useful tool to study regional GABA turnover in rats.

The gab operon is responsible for the conversion of γ-aminobutyrate (GABA) to succinate. The gab operon comprises three structural genes – gabD, gabT and gabP – that encode for a succinate semialdehyde dehydrogenase, GABA transaminase and a GABA permease respectively. There is a regulatory gene csiR, downstream of the operon, that codes for a putative transcriptional repressor and is activated when nitrogen is limiting.
Succinate-semialdehyde dehydrogenase (NADP+) (EC 1.2.1.79, succinic semialdehyde dehydrogenase (NADP+), succinyl semialdehyde dehydrogenase (NADP+), succinate semialdehyde:NADP+ oxidoreductase, NADP-dependent succinate-semialdehyde dehydrogenase, GabD) is an enzyme with systematic name succinate-semialdehyde:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
Methionine transaminase is an enzyme with systematic name L-methionine:2-oxo-acid aminotransferase. This enzyme catalyses the following chemical reaction
Gamma-aminobutyric acid aminotransferase may refer to:
4-aminobutyric acid aminotransferase may refer to:

4-Aminobutyrate aminotransferase is a protein that in humans is encoded by the ABAT gene. This gene is located in chromosome 16 at position of 13.2. This gene goes by a number of names, including, GABA transaminase, GABAT, 4-aminobutyrate transaminase, NPD009 etc. This gene is mainly and abundant located in neuronal tissues. 4-Aminobutyrate aminotransferase belongs to group of pyridoxal 5-phosphate-dependent enzyme which activates a large portion giving reaction to amino acids. ABAT is made up of two monomers of enzymes where each subunit has a molecular weight of 50kDa. It is identified that almost tierce of human synapses have GABA. GABA is a neurotransmitter that has different roles in different regions of the central and peripheral nervous systems. It can be found also in some tissues that do not have neurons. In addition, GAD and GABA-AT are responsible in regulating the concentration of GABA.












