In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. Haloarenes are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
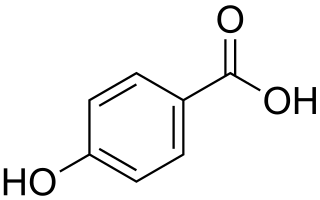
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
The crotonase family comprises mechanistically diverse proteins that share a conserved trimeric quaternary structure, the core of which consists of 4 turns of a (beta/beta/alpha)n superhelix.
In enzymology, a tetrachloroethene reductive dehalogenase is an enzyme that catalyzes the chemical reaction. This is a member of reductive dehalogenase enzyme family.
In enzymology, a 3-hydroxybenzoate 4-monooxygenase (EC 1.14.13.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a benzoate 4-monooxygenase (EC 1.14.14.92, Formerly EC 1.14.13.12) is an enzyme that catalyzes the chemical reaction
The enzyme 3-chloro-D-alanine dehydrochlorinase (EC 4.5.1.2) catalyzes the reaction
The enzyme L-2-amino-4-chloropent-4-enoate dehydrochlorinase (EC 4.5.1.4) catalyzes the reaction
In enzymology, a 4-chlorobenzoyl-CoA dehalogenase (EC 3.8.1.7) is an enzyme that catalyzes the chemical reaction
In enzymology, an alkylhalidase (EC 3.8.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a haloacetate dehalogenase (EC 3.8.1.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a haloalkane dehalogenase (EC 3.8.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-2-haloacid dehalogenase(EC 3.8.1.9), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-2-haloacid dehalogenase (EC 3.8.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-chlorobenzoate—CoA ligase is an enzyme that catalyzes the chemical reaction
The enzyme 4-hydroxybenzoyl-CoA thioesterase (EC 3.1.2.23) catalyzes the reaction
2-haloacid dehalogenase (configuration-inverting) (EC 3.8.1.10, 2-haloalkanoic acid dehalogenase, 2-haloalkanoid acid halidohydrolase, DL-2-haloacid dehalogenase, DL-2-haloacid dehalogenase (inversion of configuration), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme with systematic name (S)-2-haloacid dehalogenase (configuration-inverting). This enzyme catalyses the following chemical reaction
2-haloacid dehalogenase (configuration-retaining) (EC 3.8.1.11, 2-haloalkanoic acid dehalogenase, 2-haloalkanoid acid halidohydrolase, DL-2-haloacid dehalogenase, DL-DEXr) is an enzyme with systematic name (S)-2-haloacid dehalogenase (configuration-retaining). This enzyme catalyses the following chemical reaction
Adsorbable organic halides (AOX) is a measure of the organic halogen load at a sampling site such as soil from a land fill, water, or sewage waste. The procedure measures chlorine, bromine, and iodine as equivalent halogens, but does not measure fluorine levels in the sample.
Transhalogenation is a substitution reaction in which the halide of a halide compound is exchanged for another halide.



