
Pears are fruits produced and consumed around the world, growing on a tree and harvested in late summer into mid-autumn. The pear tree and shrub are a species of genus Pyrus, in the family Rosaceae, bearing the pomaceous fruit of the same name. Several species of pears are valued for their edible fruit and juices, while others are cultivated as trees.

Ricinus communis, the castor bean or castor oil plant, is a species of perennial flowering plant in the spurge family, Euphorbiaceae. It is the sole species in the monotypic genus, Ricinus, and subtribe, Ricininae. The evolution of castor and its relation to other species are currently being studied using modern genetic tools. It reproduces with a mixed pollination system which favors selfing by geitonogamy but at the same time can be an out-crosser by anemophily or entomophily.

Juniperus communis, the common juniper, is a species of small tree or shrub in the cypress family Cupressaceae. An evergreen conifer, it has the largest geographical range of any woody plant, with a circumpolar distribution throughout the cool temperate Northern Hemisphere.

Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, explosives and drugs. It has also been used to make bile acids, cholesterol and steroids.

Pyrus communis, the common pear, is a species of pear native to central and eastern Europe, and western Asia.

Dioscorea communis or Tamus communis is a species of flowering plant in the yam family Dioscoreaceae and is commonly known as black bryony, lady's-seal or black bindweed.
In quantum chemistry, the quantum theory of atoms in molecules (QTAIM), sometimes referred to as atoms in molecules (AIM), is a model of molecular and condensed matter electronic systems in which the principal objects of molecular structure - atoms and bonds - are natural expressions of a system's observable electron density distribution function. An electron density distribution of a molecule is a probability distribution that describes the average manner in which the electronic charge is distributed throughout real space in the attractive field exerted by the nuclei. According to QTAIM, molecular structure is revealed by the stationary points of the electron density together with the gradient paths of the electron density that originate and terminate at these points.

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.

An enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis.

Myrtus communis, the common myrtle or true myrtle, is a species of flowering plant in the myrtle family Myrtaceae. It is an evergreen shrub native to southern Europe, North Africa, Western Asia, Macaronesia, and the Indian Subcontinent, and also cultivated.

A-86929 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. It was developed as a possible treatment for Parkinson's disease, as well as for other applications such as treatment of cocaine addiction, but while it had reasonable efficacy in humans it also caused dyskinesias and has not been continued. It has mainly been used as its diacetate ester prodrug adrogolide (ABT-431), which has better bioavailability.
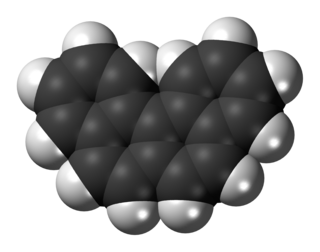
Benzo[c]phenanthrene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule consisting of the fusion of four fused benzene rings. The compound is of mainly theoretical interest but it is environmentally occurring and weakly carcinogenic.
The molecular formula C18H18O5 (molar mass: 314.33 g/mol, exact mass: 314.115424 u) may refer to:

18-Norabietane is a diterpene perhydrogenated phenanthrene derivative. It occurs in the mineral fichtelite.
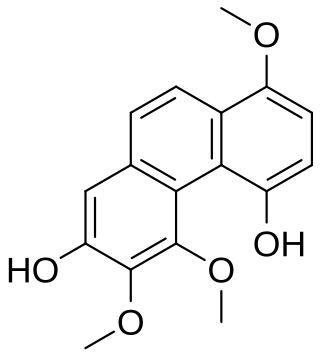
3,4,8-Trimethoxyphenanthrene-2,5-diol is one of the 17 phenanthrenes found in the extract of the stems of the orchid Dendrobium nobile.

Plicatol A is one of the three phenanthrenes that can be isolated from the stems of the orchid Dendrobium plicatile.
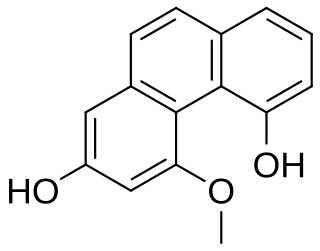
Plicatol B is one of the three phenanthrenes that can be isolated from the stems of the orchid Flickingeria fimbriata. It can also be isolated from Dendrobium densiflorum, D. loddigesii, D. moschatum, D. rotundatum and Bulbophyllum kwangtungense
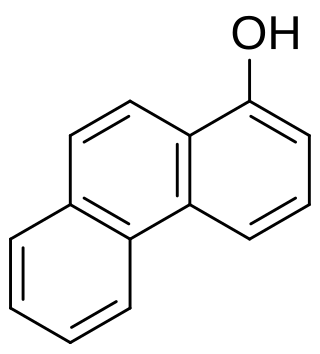
1-Hydroxyphenanthrene is a phenanthrol and a human metabolite of phenanthrene that can be detected in urine of persons exposed to PAHs.
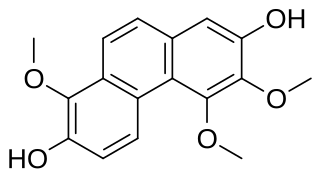
Confusarin is a phenanthrenoid found in the orchids Eria confusa and Bulbophyllum reptans. It can also be synthesized.
The Pschorr cyclization is a name reaction in organic chemistry, which was named after its discoverer, the German chemist Robert Pschorr (1868-1930). It describes the intramolecular substitution of aromatic compounds via aryldiazonium salts as intermediates and is catalyzed by copper. The reaction is a variant of the Gomberg-Bachmann reaction. The following reaction scheme shows the Pschorr cyclization for the example of phenanthrene:
















