Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. For certain infections it is given with flucytosine. It is typically given intravenously.
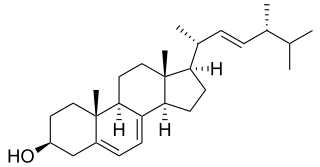
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that synthesize it have become important targets for drug discovery. In human nutrition, ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2.

Athlete's foot, known medically as tinea pedis, is a common skin infection of the feet caused by a fungus. Signs and symptoms often include itching, scaling, cracking and redness. In rare cases the skin may blister. Athlete's foot fungus may infect any part of the foot, but most often grows between the toes. The next most common area is the bottom of the foot. The same fungus may also affect the nails or the hands. It is a member of the group of diseases known as tinea.

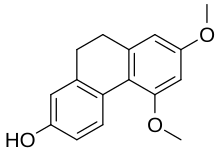
Ajoene is an organosulfur compound found in garlic (Allium sativum) extracts. It is a colorless liquid that contains sulfoxide and disulfide functional groups. The name (and pronunciation) is derived from "ajo", the Spanish word for garlic. It is found as a mixture of up to four stereoisomers, which differ in terms of the stereochemistry of the central alkene (E- vs Z-) and the chirality of the sulfoxide sulfur (R- vs S-).

Gmelina arborea,, locally known as gamhar, is a fast-growing deciduous tree in the family Lamiaceae.

Chytridiomycosis is an infectious disease in amphibians, caused by the chytrid fungi Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans. Chytridiomycosis has been linked to dramatic population declines or extinctions of amphibian species in western North America, Central America, South America, eastern Australia, east Africa (Tanzania), and Dominica and Montserrat in the Caribbean. Much of the New World is also at risk of the disease arriving within the coming years. The fungus is capable of causing sporadic deaths in some amphibian populations and 100% mortality in others. No effective measure is known for control of the disease in wild populations. Various clinical signs are seen by individuals affected by the disease. A number of options are possible for controlling this disease-causing fungus, though none has proved to be feasible on a large scale. The disease has been proposed as a contributing factor to a global decline in amphibian populations that apparently has affected about 30% of the amphibian species of the world. Some research found evidence insufficient for linking chytrid fungi and chytridiomycosis to global amphibian declines, but more recent research establishes a connection and attributes the spread of the disease to its transmission through international trade routes into native ecosystems.

Dermatophytosis, also known as ringworm, is a fungal infection of the skin. Typically it results in a red, itchy, scaly, circular rash. Hair loss may occur in the area affected. Symptoms begin four to fourteen days after exposure. Multiple areas can be affected at a given time.
Polyene antimycotics, sometimes referred to as polyene antibiotics, are a class of antimicrobial polyene compounds that target fungi. These polyene antimycotics are typically obtained from some species of Streptomyces bacteria. Previously, polyenes were thought to bind to ergosterol in the fungal cell membrane and thus weakening it and causing leakage of K+ and Na+ ions, which could contribute to fungal cell death. However, more detailed studies of polyene molecular properties have challenged this model suggesting that polyenes instead bind and extract ergosterol directly from the cellular membrane thus disrupting the many cellular functions ergosterols perform. Amphotericin B, nystatin, and natamycin are examples of polyene antimycotics. They are a subgroup of macrolides.

Sporothrix schenckii, a fungus that can be found worldwide in the environment, is named for medical student Benjamin Schenck, who in 1896 was the first to isolate it from a human specimen. The species is present in soil as well as in and on living and decomposing plant material such as peat moss. It can infect humans as well as animals and is the causative agent of sporotrichosis, commonly known as "rose handler's disease." The most common route of infection is the introduction of spores to the body through a cut or puncture wound in the skin. Infection commonly occurs in otherwise healthy individuals but is rarely life-threatening and can be treated with antifungals. In the environment it is found growing as filamentous hyphae. In host tissue it is found as a yeast. The transition between the hyphal and yeast forms is temperature dependent making S. schenckii a thermally dimorphic fungus.
Rhizopus microsporus is a fungal plant pathogen infecting maize, sunflower, and rice.

Mycocentrospora acerina is a deuteromycete fungus that is a plant pathogen.
Achlya klebsiana is a plant pathogen. Studies say that this fungi potentially poses threats against fish in the Nile.
Colletotrichum fragariae is a fungal plant pathogen infecting strawberries. It is not a well known fungus, and there are many similar fungi that are related to it. It is part of the Colletotrichum genus. It is a pathogen that occurs in strawberries. It leads to the disease known as anthracnose. This is typically at the crown of the strawberry, which is why it is often called crown rot. It is also known as the Anthracnose Crown rot. The fungus also infects leaves and is known as leaf spot, which is common among all Colletotrichum. This is not as common in the fragariae, as it is more common in the crown. This fungus is also better at infecting younger strawberries/seedlings. The most common way to control this disease is fungicides that are harmful to the environment. There have been studies done to see if the fungus infects other hosts but other than some weeds, it is very specific to Strawberries.

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:
A lipopeptide is a molecule consisting of a lipid connected to a peptide. They are able to self-assemble into different structures. Many bacteria produce these molecules as a part of their metabolism, especially those of the genus Bacillus, Pseudomonas and Streptomyces. Certain lipopeptides are used as antibiotics. Due to the structural and molecular properties such as the fatty acid chain, it poses the effect of weakening the cell function or destroying the cell.Other lipopeptides are toll-like receptor agonists. Certain lipopeptides can have strong antifungal and hemolytic activities. It has been demonstrated that their activity is generally linked to interactions with the plasma membrane, and sterol components of the plasma membrane could play a major role in this interaction. It is a general trend that adding a lipid group of a certain length to a lipopeptide will increase its bactericidal activity. Lipopeptides with a higher amount of carbon atoms, for example 14 or 16, in its lipid tail will typically have antibacterial activity as well as anti-fungal activity.Therefore, an increase in the alkyl chain can make lipopeptides soluble in water.As well, it opens the cell membrane of the bacteria, so antimicrobial activity can take place.

Fonsecaea pedrosoi is a fungal species in the family Herpotrichiellaceae, and the major causative agent of chromoblastomycosis. This species is commonly found in tropical and sub-tropical regions, especially in South America, where it grows as a soil saprotroph. Farming activities in the endemic zone are a risk factor for the development of chromoblastomycosis.

Guarianthe aurantiaca is a species of orchid. It is widespread across much of Mexico, south to Costa Rica. The diploid chromosome number of G. aurantiaca has been determined as 2n = 40.

Trifolin is a chemical compound. It is the kaempferol 3-galactoside. It can be found in Camptotheca acuminata, in Euphorbia condylocarpa or in Consolida oliveriana.

Fusarium mangiferae is a fungal plant pathogen that infects mango trees. Its aerial mycelium is white and floccose. Conidiophores on aerial mycelium originating erect and prostrate from substrate; they are sympodially branched bearing mono and polyphialides. Polyphialides have 2–5 conidiogenous openings. Phialides on the aerial conidiophores mono- and polyphialidic. Sterile hyphae are absent. Microconidia are variable in shape, obovoid conidia are the most abundant type, oval to allantoid conidia occurring occasionally. Microconidia mostly 0-septate with 1-septate conidia occurring less abundantly. Sporodochia are present. Macroconidia are long and slender, usually 3–5 septate. Chlamydospores are absent.
Microsporum nanum is a pathogenic fungus in the family Arthrodermataceae. It is a type of dermatophyte that causes infection in dead keratinized tissues such as skin, hair, and nails. Microsporum nanum is found worldwide and is both zoophilic and geophilic. Animals such as pigs and sheep are the natural hosts for the fungus; however, infection of humans is also possible. Majority of the human cases reported are associated with pig farming. The fungus can invade the skin of the host; if it is scratched off by the infected animal, the fungus is still capable of reproducing in soil.