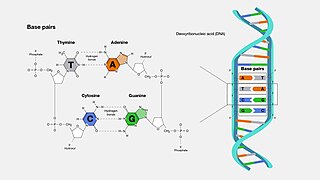
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA and RNA. Dictated by specific hydrogen bonding patterns, "Watson–Crick" base pairs allow the DNA helix to maintain a regular helical structure that is subtly dependent on its nucleotide sequence. The complementary nature of this based-paired structure provides a redundant copy of the genetic information encoded within each strand of DNA. The regular structure and data redundancy provided by the DNA double helix make DNA well suited to the storage of genetic information, while base-pairing between DNA and incoming nucleotides provides the mechanism through which DNA polymerase replicates DNA and RNA polymerase transcribes DNA into RNA. Many DNA-binding proteins can recognize specific base-pairing patterns that identify particular regulatory regions of genes.
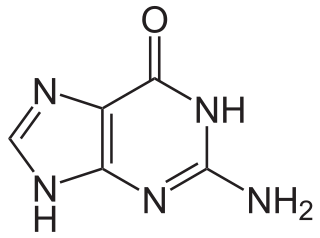
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine. In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine.

Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.
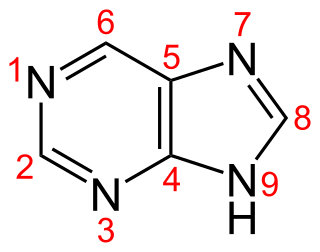
Purine is a heterocyclic aromatic organic compound that consists of two rings fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature.
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

Nucleobases are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called primary or canonical. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, creating a more stable bond to thymine.

Carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Over 1,100 identified carotenoids can be further categorized into two classes – xanthophylls and carotenes.

Luminol (C8H7N3O2) is a chemical that exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. Luminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents, but insoluble in water.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.
Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Ribose 5-phosphate (R5P) is both a product and an intermediate of the pentose phosphate pathway. The last step of the oxidative reactions in the pentose phosphate pathway is the production of ribulose 5-phosphate. Depending on the body's state, ribulose 5-phosphate can reversibly isomerize to ribose 5-phosphate. Ribulose 5-phosphate can alternatively undergo a series of isomerizations as well as transaldolations and transketolations that result in the production of other pentose phosphates as well as fructose 6-phosphate and glyceraldehyde 3-phosphate.
In enzymology, a diaminohydroxyphosphoribosylaminopyrimidine deaminase (EC 3.5.4.26) is an enzyme that catalyzes the chemical reaction

In enzymology, a 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase is an enzyme that catalyzes the chemical reaction

IDRA-21 is a positive allosteric modulator of the AMPA receptor and a benzothiadiazine derivative. It is a chiral molecule, with (+)-IDRA-21 being the active form.

1-Testosterone, also known as δ1-dihydrotestosterone (δ1-DHT), as well as dihydroboldenone, is a synthetic anabolic–androgenic steroid (AAS) and a 5α-reduced derivative of boldenone (Δ1-testosterone). It differs from testosterone by having a 1(2)-double bond instead of a 4(5)-double bond in its A ring. It was legally sold online in the United States until 2005, when it was reclassified as a Schedule III drug.
2-amino-5-formylamino-6-ribosylaminopyrimidin-4(3H)-one 5'-monophosphate deformylase (EC 3.5.1.102, ArfB) is an enzyme with systematic name 2-amino-5-formylamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one amidohydrolase. This enzyme catalyses the following chemical reaction

2,5-diamino-6-hydroxy-4-(5-phosphoribosylamino)pyrimidine is a metabolite in the purine metabolism, formed by the hydrolysis of GTP by GTP cyclohydrolase II. Alternatively two separate enzymes can carry out this reaction, initially GTP cyclohydrolase IIa hydrolyses the 8,9 bond to form 2-Amino-5-formylamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one, followed by de-formylation by 2-amino-5-formylamino-6-ribosylaminopyrimidin-4(3H)-one 5'-monophosphate deformylase. 2,5-diamino-6-hydroxy-4-(5-phosphoribosylamino)pyrimidine is deaminated by Diaminohydroxyphosphoribosylaminopyrimidine deaminase to form 5-amino-6-(5-phosphoribosylamino)uracil.
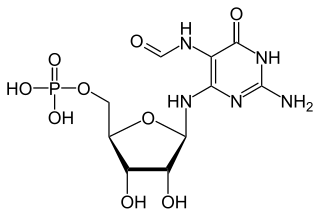
2-Amino-5-formylamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one is a metabolite in the riboflavin biosynthesis pathway. It is formed from GTP by the enzyme GTP cyclohydrolase IIa which catalyzes the hydrolysis of the 8,9 bond in the guanine group and loss of the beta and gamma phosphate groups. The molecule is deformylated by 2-amino-5-formylamino-6-ribosylaminopyrimidin-4(3H)-one 5'-monophosphate deformylase as the second step in the archaeal riboflavin biosynthetic pathway.















