Contents
 | |
 | |
| Names | |
|---|---|
| IUPAC name (1R,2R,5S,8S,9S,12S,13R,14S,15S,16R,17S,20S,21S,24S)-12,17-dihydroxy-3,8,12,17,21,25-hexamethyl-6,23-dioxaheptacyclo[13.9.2.01,16.02,14.04,13.05,9.020,24]hexacosa-3,25-diene-7,22-dione | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C30H40O6 | |
| Molar mass | 496.635 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
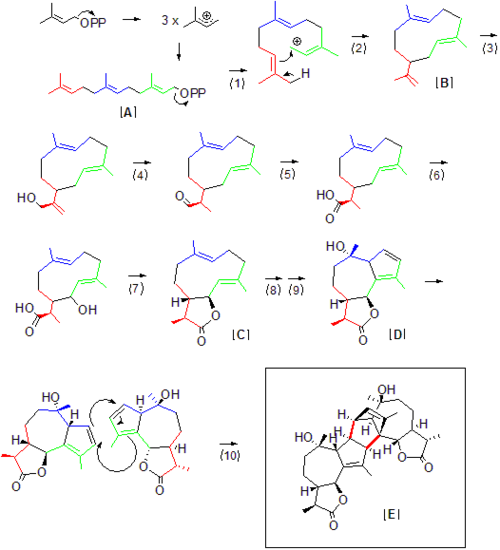
Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. [1] The compound shows biological activity and has shown promise as an anti-inflammatory agent, [2] and should not be confused with thujone, a neurotoxin also found in Artemisia absinthium .