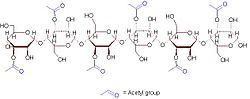
 A representative 6-sugar fragment of acemannan | |
| Names | |
|---|---|
| IUPAC name (2S,3S,4R,5S,6S)-6-[(2R,3R,4R,5S,6R)-6-[(2R,3S,4R,5S,6R)-5-acetamido-6-[(2R,3R,4R,5S,6R)-4-acetyloxy-6-[(2R,3R,4R,5S,6R)-4-acetyloxy-6-[(2R,3R,4R,5S,6S)-4-acetyloxy-5-hydroxy-2-(hydroxymethyl)-6-methoxyoxan-3-yl]oxy-5-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-5-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-acetyloxy-5-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-acetyloxy-3-[(2R,3S,4R,5R,6R)-4-acetyloxy-5-[(2R,3S,4R,5R,6R)-4-acetyloxy-3-hydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5-hydroxyoxane-2-carboxylate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.122.396 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C66H100NO49 | |
| Molar mass | 1691.484 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Acemannan is a D-isomer mucopolysaccharide in aloe vera leaves. This compound has potential immunostimulant, [1] antiviral, antineoplastic, and gastrointestinal properties. [2]