The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C. Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
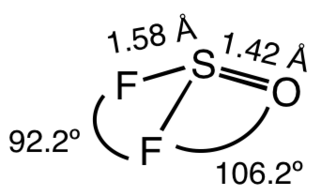
Thionyl fluoride is the inorganic compound with the formula SOF
2. This colourless gas is mainly of theoretical interest, but it is a product of the degradation of sulfur hexafluoride, an insulator in electrical equipment. The molecule adopts a distorted pyramidal structure, with Cs symmetry. The S-O and S-F distances are 1.42 and 1.58 Å, respectively. The O-S-F and F-S-F angles are 106.2 and 92.2°, respectively. Thionyl chloride and thionyl bromide have similar structures, although these compounds are liquid at room temperature. Mixed halides are also known, such as SOClF, thionyl chloride fluoride.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
Dichloromethane dehalogenase (EC 4.5.1.3; systematic name dichloromethane chloride-lyase (adding H2O; chloride-hydrolysing; formaldehyde-forming)) is a lyase enzyme that generates formaldehyde.
The enzyme L-2-amino-4-chloropent-4-enoate dehydrochlorinase (EC 4.5.1.4) catalyzes the reaction
In enzymology, a 4-chlorobenzoate dehalogenase (EC 3.8.1.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-chlorobenzoyl-CoA dehalogenase (EC 3.8.1.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a haloacetate dehalogenase (EC 3.8.1.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a haloalkane dehalogenase (EC 3.8.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-2-haloacid dehalogenase(EC 3.8.1.9), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-2-haloacid dehalogenase (EC 3.8.1.2) is an enzyme that catalyzes the chemical reaction
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment.
2-haloacid dehalogenase (configuration-inverting) (EC 3.8.1.10, 2-haloalkanoic acid dehalogenase, 2-haloalkanoid acid halidohydrolase, DL-2-haloacid dehalogenase, DL-2-haloacid dehalogenase (inversion of configuration), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme with systematic name (S)-2-haloacid dehalogenase (configuration-inverting). This enzyme catalyses the following chemical reaction
2-haloacid dehalogenase (configuration-retaining) (EC 3.8.1.11, 2-haloalkanoic acid dehalogenase, 2-haloalkanoid acid halidohydrolase, DL-2-haloacid dehalogenase, DL-DEXr) is an enzyme with systematic name (S)-2-haloacid dehalogenase (configuration-retaining). This enzyme catalyses the following chemical reaction
Transhalogenation is a substitution reaction in which the halide of a halide compound is exchanged for another halide.






