Related Research Articles

Transfer RNA is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length. In a cell, it provides the physical link between the genetic code in messenger RNA (mRNA) and the amino acid sequence of proteins, carrying the correct sequence of amino acids to be combined by the protein-synthesizing machinery, the ribosome. Each three-nucleotide codon in mRNA is complemented by a three-nucleotide anticodon in tRNA. As such, tRNAs are a necessary component of translation, the biological synthesis of new proteins in accordance with the genetic code.

An aminoacyl-tRNA synthetase, also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA. It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code.

Aminoacyl-tRNA is tRNA to which its cognate amino acid is chemically bonded (charged). The aa-tRNA, along with particular elongation factors, deliver the amino acid to the ribosome for incorporation into the polypeptide chain that is being produced during translation.

In enzymology, an arginine—tRNA ligase is an enzyme that catalyzes the chemical reaction
Aspartate—tRNAAsn ligase is an enzyme with systematic name L-aspartate:tRNAAsx ligase (AMP-forming). This enzyme catalyses the following chemical reaction
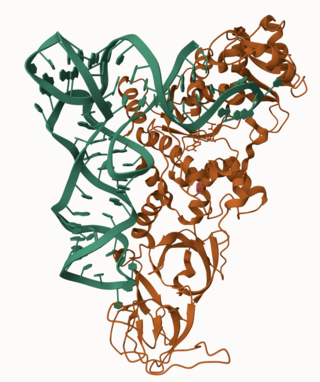
Glutamine—tRNA ligase or glutaminyl-tRNA synthetase (GlnRS) is an aminoacyl-tRNA synthetase, also called tRNA-ligase. is an enzyme that attaches the amino acid glutamine onto its cognate tRNA.

In enzymology, a phenylalanine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a threonine-tRNA ligase is an enzyme that catalyzes the chemical reaction
Tyrosine—tRNA ligase, also known as tyrosyl-tRNA synthetase is an enzyme that is encoded by the gene YARS. Tyrosine—tRNA ligase catalyzes the chemical reaction

Tyrosyl-tRNA synthetase, cytoplasmic, also known as Tyrosine-tRNA ligase, is an enzyme that in humans is encoded by the YARS gene.
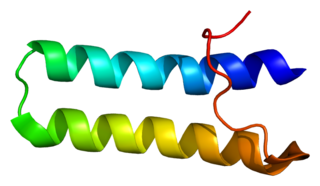
Bifunctional aminoacyl-tRNA synthetase is an enzyme that in humans is encoded by the EPRS gene.
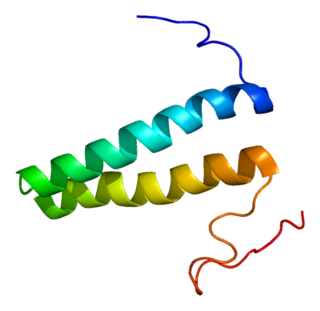
Methionyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the MARS gene.

Isoleucyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the IARS1 gene.

Asparaginyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the NARS gene.

Probable histidyl-tRNA synthetase, mitochondrial is an enzyme that in humans is encoded by the HARS2 gene.
Amino acid activation refers to the attachment of an amino acid to its respective transfer RNA (tRNA). The reaction occurs in the cell cytosol and consists of two steps: first, the enzyme aminoacyl tRNA synthetase catalyzes the binding of adenosine triphosphate (ATP) to a corresponding amino acid, forming a reactive aminoacyl adenylate intermediate and releasing inorganic pyrophosphate (PPi). Subsequently, aminoacyl tRNA synthetase binds the AMP-amino acid to a tRNA molecule, releasing AMP and attaching the amino acid to the tRNA. The resulting aminoacyl-tRNA is said to be charged.
The aminoacyl-tRNA synthetases catalyse the attachment of an amino acid to its cognate transfer RNA molecule in a highly specific two-step reaction. These proteins differ widely in size and oligomeric state, and have limited sequence homology. The 20 aminoacyl-tRNA synthetases are divided into two classes, I and II. Class I aminoacyl-tRNA synthetases contain a characteristic Rossmann fold catalytic domain and are mostly monomeric. Class II aminoacyl-tRNA synthetases share an anti-parallel beta-sheet fold flanked by alpha-helices, and are mostly dimeric or multimeric, containing at least three conserved regions. However, tRNA binding involves an alpha-helical structure that is conserved between class I and class II synthetases. In reactions catalysed by the class I aminoacyl-tRNA synthetases, the aminoacyl group is coupled to the 2'-hydroxyl of the tRNA, while, in class II reactions, the 3'-hydroxyl site is preferred. The synthetases specific for arginine, cysteine, glutamic acid, glutamine, isoleucine, leucine, methionine, tyrosine, tryptophan and valine belong to class I synthetases; these synthetases are further divided into three subclasses, a, b and c, according to sequence homology. The synthetases specific for alanine, asparagine, aspartic acid, glycine, histidine, lysine, phenylalanine, proline, serine, and threonine belong to class-II synthetases.

The B3/B4 domain, is found in tRNA synthetase beta subunits, as well as in some non-tRNA synthetase proteins.

In molecular biology, YqeY is a type of protein domain of unknown function. It is thought to have a role in protein synthesis, facilitating the production of charged transfer RNA used in the process of translating mRNA into protein. It is present as a domain of glutaminyl-tRNA synthetase (GlnRS) in almost all eukaryotes.
Dino Moras, born on 23 November 1944, is a French biochemist, research director at the CNRS and co-director of the Institute of Genetics and Molecular and Cellular Biology (IGBMC) in Illkirch-Graffenstaden until 2010.
References
- ↑ Delarue M, Moras D, Poch O, Eriani G, Gangloff J (1990). "Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs". Nature. 347 (6289): 203–206. Bibcode:1990Natur.347..203E. doi:10.1038/347203a0. PMID 2203971. S2CID 4324290.
- ↑ Moras D, Konno M, Shimada A, Nureki O, Tateno M, Yokoyama S, Sugiura I, Ugaji-Yoshikawa Y, Kuwabara S, Lorber B, Giege R (2000). "The 2.0 A crystal structure of Thermus thermophilus methionyl-tRNA synthetase reveals two RNA-binding modules". Structure. 8 (2): 197–208. doi: 10.1016/S0969-2126(00)00095-2 . PMID 10673435.
- ↑ Perona JJ, Steitz TA, Rould MA (1993). "Structural basis for transfer RNA aminoacylation by Escherichia coli glutaminyl-tRNA synthetase". Biochemistry. 32 (34): 8758–8771. doi:10.1021/bi00085a006. PMID 8364025.
- ↑ Delarue M, Moras D (1993). "The aminoacyl-tRNA synthetase family: modules at work". BioEssays. 15 (10): 675–687. doi:10.1002/bies.950151007. PMID 8274143. S2CID 35612984.
- ↑ Schimmel P (1991). "Classes of aminoacyl-tRNA synthetases and the establishment of the genetic code". Trends Biochem. Sci. 16 (1): 1–3. doi:10.1016/0968-0004(91)90002-D. PMID 2053131.
- ↑ Cusack S, Leberman R, Hartlein M (1991). "Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases". Nucleic Acids Res. 19 (13): 3489–3498. doi:10.1093/nar/19.13.3489. PMC 328370 . PMID 1852601.
- ↑ Bairoch A (2004). "List of aminoacyl-tRNA synthetases".
{{cite journal}}: Cite journal requires|journal=(help)