
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.
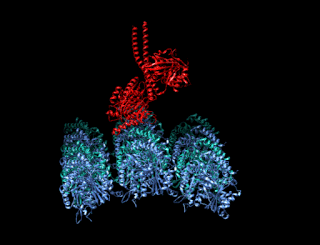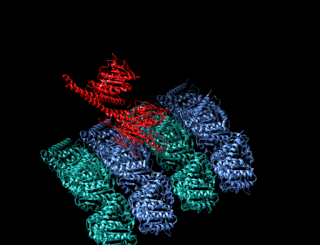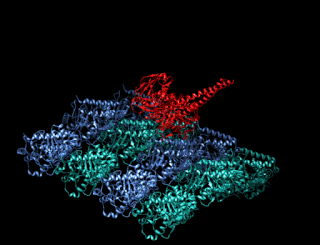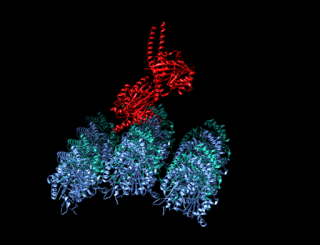
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport.

Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport.

In molecular biology, an axoneme, also called an axial filament, is the microtubule-based cytoskeletal structure that forms the core of a cilium or flagellum. Cilia and flagella are found on many cells, organisms, and microorganisms, to provide motility. The axoneme serves as the "skeleton" of these organelles, both giving support to the structure and, in some cases, the ability to bend. Though distinctions of function and length may be made between cilia and flagella, the internal structure of the axoneme is common to both.
In cell biology, microtubule-associated proteins (MAPs) are proteins that interact with the microtubules of the cellular cytoskeleton. MAPs are integral to the stability of the cell and its internal structures and the transport of components within the cell.

Axonal transport, also called axoplasmic transport or axoplasmic flow, is a cellular process responsible for movement of mitochondria, lipids, synaptic vesicles, proteins, and other organelles to and from a neuron's cell body, through the cytoplasm of its axon called the axoplasm. Since some axons are on the order of meters long, neurons cannot rely on diffusion to carry products of the nucleus and organelles to the ends of their axons. Axonal transport is also responsible for moving molecules destined for degradation from the axon back to the cell body, where they are broken down by lysosomes.

Motor proteins are a class of molecular motors that can move along the cytoskeleton of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.

Dynactin is a 23 subunit protein complex that acts as a co-factor for the microtubule motor cytoplasmic dynein-1. It is built around a short filament of actin related protein-1 (Arp1).

CAP-Gly domain containing linker protein 1, also known as CLIP1, is a protein which in humans is encoded by the CLIP1 gene.

Bicaudal D cargo adaptor 1 is a protein that in humans is encoded by the BICD1 gene.

Ronald David Vale ForMemRS is an American biochemist and cell biologist. He is a professor at the Department of Cellular and Molecular Pharmacology, University of California, San Francisco. His research is focused on motor proteins, particularly kinesin and dynein. He was awarded the Canada Gairdner International Award for Biomedical Research in 2019, the Shaw Prize in Life Science and Medicine in 2017 together with Ian Gibbons, and the Albert Lasker Award for Basic Medical Research in 2012 alongside Michael Sheetz and James Spudich. He is a fellow of the American Academy of Arts and Sciences and a member of the National Academy of Sciences. He was the president of the American Society for Cell Biology in 2012. He has also been an investigator at the Howard Hughes Medical Institute since 1995. In 2019, Vale was named executive director of the Janelia Research Campus and a vice president of HHMI; his appointment began in early 2020.

Intracellular transport is the movement of vesicles and substances within a cell. Intracellular transport is required for maintaining homeostasis within the cell by responding to physiological signals. Proteins synthesized in the cytosol are distributed to their respective organelles, according to their specific amino acid’s sorting sequence. Eukaryotic cells transport packets of components to particular intracellular locations by attaching them to molecular motors that haul them along microtubules and actin filaments. Since intracellular transport heavily relies on microtubules for movement, the components of the cytoskeleton play a vital role in trafficking vesicles between organelles and the plasma membrane by providing mechanical support. Through this pathway, it is possible to facilitate the movement of essential molecules such as membrane‐bounded vesicles and organelles, mRNA, and chromosomes.

Microtubule plus-end/positive-end tracking proteins or +TIPs are a type of microtubule associated protein (MAP) which accumulate at the plus ends of microtubules. +TIPs are arranged in diverse groups which are classified based on their structural components; however, all classifications are distinguished by their specific accumulation at the plus end of microtubules and their ability to maintain interactions between themselves and other +TIPs regardless of type. +TIPs can be either membrane bound or cytoplasmic, depending on the type of +TIPs. Most +TIPs track the ends of extending microtubules in a non-autonomous manner.

Marileen Dogterom is a Dutch biophysicist and professor at the Kavli Institute of Nanoscience at Delft University of Technology. She published in Science, Cell, and Nature and is notable for her research of the cell cytoskeleton. For this research, she was awarded the 2018 Spinoza Prize.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.
Casper Hoogenraad is a Dutch Cell Biologist who specializes in molecular neuroscience. The focus of his research is the basic molecular and cellular mechanisms that regulate the development and function of the brain. As of January 2020, he serves as Vice President of Neuroscience at Genentech Research and Early Development.
Jennifer L. Ross is an American physicist who is Professor and Chair of the Department of Physics at Syracuse University. Her research considers active biological condensed matter physics. She was elected fellow of the American Physical Society in 2018 and American Association for the Advancement of Science in 2022.

Erika L F. Holzbaur is an American biologist who is the William Maul Measey Professor of Physiology at University of Pennsylvania Perelman School of Medicine. Her research considers the dynamics of organelle motility along cytoskeleton of cells. She is particularly interested in the molecular mechanisms that underpin neurodegenerative diseases.
J. Richard McIntosh is a Distinguished Professor Emeritus in Molecular, Cellular, and Developmental Biology at the University of Colorado Boulder. McIntosh first graduated from Harvard with a BA in Physics in 1961, and again with a Ph.D. in Biophysics in 1968. He began his teaching career at Harvard but has spent most of his career at the University of Colorado Boulder. At the University of Colorado Boulder, McIntosh taught biology courses at both the undergraduate and graduate levels. Additionally, he created an undergraduate course in the biology of cancer towards the last several years of his teaching career. McIntosh's research career looks at a variety of things, including different parts of mitosis, microtubules, and motor proteins.















