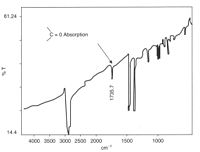
Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to the wavenumber in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO)

Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.
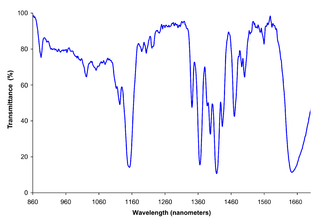
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum. Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology, and neurology. There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and astronomy.

Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a method of thermal analysis in which the mass of a sample is measured over time as the temperature changes. This measurement provides information about physical phenomena, such as phase transitions, absorption, adsorption and desorption; as well as chemical phenomena including chemisorptions, thermal decomposition, and solid-gas reactions.

According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When such quanta of electromagnetic radiation are emitted or absorbed by an atom or molecule, energy of the radiation changes the state of the atom or molecule from an initial state to a final state. An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are characteristic of a particular transition from initial to final state in a substance.

Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroys paintings and other artifacts. It is, however, partly responsible for remineralization of biomass and is used intentionally in some disinfection technologies. Photodegradation does not apply to how materials may be aged or degraded via infrared light or heat, but does include degradation in all of the ultraviolet light wavebands.

Spectrochemistry is the application of spectroscopy in several fields of chemistry. It includes analysis of spectra in chemical terms, and use of spectra to derive the structure of chemical compounds, and also to qualitatively and quantitively analyze their presence in the sample. It is a method of chemical analysis that relies on the measurement of wavelengths and intensity of electromagnetic radiation.

Forensic materials engineering, a branch of forensic engineering, focuses on the material evidence from crime or accident scenes, seeking defects in those materials which might explain why an accident occurred, or the source of a specific material to identify a criminal. Many analytical methods used for material identification may be used in investigations, the exact set being determined by the nature of the material in question, be it metal, glass, ceramic, polymer or composite. An important aspect is the analysis of trace evidence such as skid marks on exposed surfaces, where contact between dissimilar materials leaves material traces of one left on the other. Provided the traces can be analysed successfully, then an accident or crime can often be reconstructed. Another aim will be to determine the cause of a broken component using the technique of fractography.

Forensic polymer engineering is the study of failure in polymeric products. The topic includes the fracture of plastic products, or any other reason why such a product fails in service, or fails to meet its specification. The subject focuses on the material evidence from crime or accident scenes, seeking defects in those materials that might explain why an accident occurred, or the source of a specific material to identify a criminal. Many analytical methods used for polymer identification may be used in investigations, the exact set being determined by the nature of the polymer in question, be it thermoset, thermoplastic, elastomeric or composite in nature.
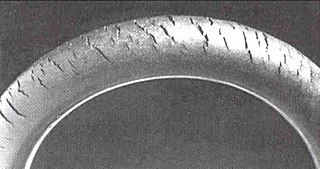
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those products owing to preventive measures.
In polymers, such as plastics, thermal degradation refers to a type of polymer degradation where damaging chemical changes take place at elevated temperatures, without the simultaneous involvement of other compounds such as oxygen. Simply put, even in the absence of air, polymers will begin to degrade if heated high enough. It is distinct from thermal-oxidation, which can usually take place at less elevated temperatures.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.
Accelerated photo-ageing of polymers in SEPAP units is the controlled polymer degradation and polymer coating degradation under lab or natural conditions.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.

Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time.

Twin-wall plastic, specifically twin-wall polycarbonate, is an extruded multi-wall polymer product created for applications where its strength, thermally insulative properties, and moderate cost are ideal. Polycarbonate, which is most commonly formed through the reaction of Bisphenol A and Carbonyl Chloride, is an extremely versatile material. It is significantly lighter than glass, while managing to be stronger, more flexible, and more impact resistant. Twin-wall polycarbonate is used most commonly for green houses, where it can support itself in a structurally sound configuration, limit the amount of UV light due to its nominal translucence, and can withstand the rigors of daily abuse in an outdoor environment. The stagnant air in the cellular space between sheets provides insulation, and additional cell layers can be extruded to enhance insulative properties at the cost of light transmission.
The technique of vibrational analysis with scanning probe microscopy allows probing vibrational properties of materials at the submicrometer scale, and even of individual molecules. This is accomplished by integrating scanning probe microscopy (SPM) and vibrational spectroscopy. This combination allows for much higher spatial resolution than can be achieved with conventional Raman/FTIR instrumentation. The technique is also nondestructive, requires non-extensive sample preparation, and provides more contrast such as intensity contrast, polarization contrast and wavelength contrast, as well as providing specific chemical information and topography images simultaneously.

Spectroelectrochemistry (SEC) is a set of multi-response analytical techniques in which complementary chemical information is obtained in a single experiment. Spectroelectrochemistry provides a whole vision of the phenomena that take place in the electrode process. The first spectroelectrochemical experiment was carried out by Theodore Kuwana, PhD, in 1964.