A polymer is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Forensic engineering has been defined as "the investigation of failures—ranging from serviceability to catastrophic—which may lead to legal activity, including both civil and criminal". The forensic engineering field is very broad in terms of the many disciplines that it covers, investigations that use forensic engineering are case of environmental damages to structures, system failures of machines, explosions, electrical, fire point of origin, vehicle failures and many more.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.

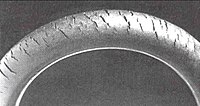
An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more parts, forming a seal at the interface.
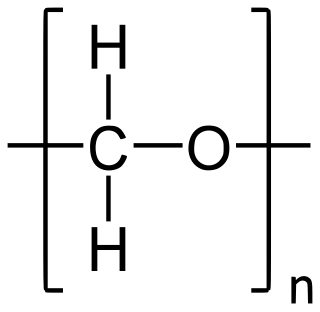
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. As with many other synthetic polymers, it is produced by different chemical firms with slightly different formulas and sold variously by such names as Delrin, Kocetal, Ultraform, Celcon, Ramtal, Duracon, Kepital, Polypenco, Tenac and Hostaform.
Failure causes are defects in design, process, quality, or part application, which are the underlying cause of a failure or which initiate a process which leads to failure. Where failure depends on the user of the product or process, then human error must be considered.
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds are cleaved with ozone. Multiple carbon–carbon bond are replaced by carbonyl groups, such as aldehydes, ketones, and carboxylic acids. The reaction is predominantly applied to alkenes, but alkynes and azo compounds are also susceptible to cleavage. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.
Failure analysis is the process of collecting and analyzing data to determine the cause of a failure, often with the goal of determining corrective actions or liability. According to Bloch and Geitner, ”machinery failures reveal a reaction chain of cause and effect… usually a deficiency commonly referred to as the symptom…”. Failure analysis can save money, lives, and resources if done correctly and acted upon. It is an important discipline in many branches of manufacturing industry, such as the electronics industry, where it is a vital tool used in the development of new products and for the improvement of existing products. The failure analysis process relies on collecting failed components for subsequent examination of the cause or causes of failure using a wide array of methods, especially microscopy and spectroscopy. Nondestructive testing (NDT) methods are valuable because the failed products are unaffected by analysis, so inspection sometimes starts using these methods.

Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments. The chemical environment that causes SCC for a given alloy is often one which is only mildly corrosive to the metal. Hence, metal parts with severe SCC can appear bright and shiny, while being filled with microscopic cracks. This factor makes it common for SCC to go undetected prior to failure. SCC often progresses rapidly, and is more common among alloys than pure metals. The specific environment is of crucial importance, and only very small concentrations of certain highly active chemicals are needed to produce catastrophic cracking, often leading to devastating and unexpected failure.
In materials science, environmental stress fracture or environment assisted fracture is the generic name given to premature failure under the influence of tensile stresses and harmful environments of materials such as metals and alloys, composites, plastics and ceramics.

Embrittlement is a significant decrease of ductility of a material, which makes the material brittle. Embrittlement is used to describe any phenomena where the environment compromises a stressed material's mechanical performance, such as temperature or environmental composition. This is oftentimes undesirable as brittle fracture occurs quicker and can much more easily propagate than ductile fracture, leading to complete failure of the equipment. Various materials have different mechanisms of embrittlement, therefore it can manifest in a variety of ways, from slow crack growth to a reduction of tensile ductility and toughness.

Fractography is the study of the fracture surfaces of materials. Fractographic methods are routinely used to determine the cause of failure in engineering structures, especially in product failure and the practice of forensic engineering or failure analysis. In material science research, fractography is used to develop and evaluate theoretical models of crack growth behavior.
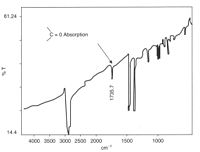
Applied spectroscopy is the application of various spectroscopic methods for the detection and identification of different elements or compounds to solve problems in fields like forensics, medicine, the oil industry, atmospheric chemistry, and pharmacology.

Forensic materials engineering, a branch of forensic engineering, focuses on the material evidence from crime or accident scenes, seeking defects in those materials which might explain why an accident occurred, or the source of a specific material to identify a criminal. Many analytical methods used for material identification may be used in investigations, the exact set being determined by the nature of the material in question, be it metal, glass, ceramic, polymer or composite. An important aspect is the analysis of trace evidence such as skid marks on exposed surfaces, where contact between dissimilar materials leaves material traces of one left on the other. Provided the traces can be analysed successfully, then an accident or crime can often be reconstructed. Another aim will be to determine the cause of a broken component using the technique of fractography.

Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those products owing to preventive measures.

Environmental Stress Cracking (ESC) is one of the most common causes of unexpected brittle failure of thermoplastic polymers known at present. According to ASTM D883, stress cracking is defined as "an external or internal crack in a plastic caused by tensile stresses less than its short-term mechanical strength". This type of cracking typically involves brittle cracking, with little or no ductile drawing of the material from its adjacent failure surfaces. Environmental stress cracking may account for around 15-30% of all plastic component failures in service. This behavior is especially prevalent in glassy, amorphous thermoplastics. Amorphous polymers exhibit ESC because of their loose structure which makes it easier for the fluid to permeate into the polymer. Amorphous polymers are more prone to ESC at temperature higher than their glass transition temperature (Tg) due to the increased free volume. When Tg is approached, more fluid can permeate into the polymer chains.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.
Maciej S. Kumosa is a materials scientist and academic. He is a professor in the Department of Mechanical and Materials Engineering at the University of Denver, and the Director of the Center for Novel High Voltage/Temperature Materials and Structures (HVT).