
Forensic science, also known as criminalistics, is the application of science principles and methods to support legal decision-making in matters of criminal and civil law.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc., which have been dissolved into liquid solutions.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC–MS include drug detection, fire investigation, environmental analysis, explosives investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.

A crime scene is any location that may be associated with a committed crime. Crime scenes contain physical evidence that is pertinent to a criminal investigation. This evidence is collected by crime scene investigators (CSI) and law enforcement. The location of a crime scene can be the place where the crime took place or can be any area that contains evidence from the crime itself. Scenes are not only limited to a location, but can be any person, place, or object associated with the criminal behaviours that occurred.
Accelerants, or accelerators, are substances that increase the rate of a natural or artificial chemical process. They play a major role in chemistry, as most chemical reactions can be hastened with an accelerant. Understanding accelerants is crucial in forensic science, engineering, and other fields where controlled chemical reactions are essential. Accelerants function by either altering a chemical bond, speeding up a chemical process, or changing the reaction conditions. Unlike catalysts, accelerants may be consumed during the process.

Trace evidence occurs when objects make contact, and material is transferred. This type of evidence is usually not visible to the naked eye and requires specific tools and techniques to be located and obtained. Due to this, trace evidence is often overlooked, and investigators must be trained to detect it. When it comes to an investigation trace evidence can come in many different forms and is found in a wide variety of cases. This evidence can link a victim to suspects and a victim or suspect to the crime scene.
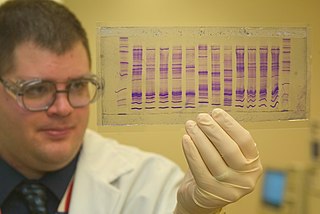
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide array of methods and instruments to help identify unknown substances. These include high-performance liquid chromatography, gas chromatography-mass spectrometry, atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and thin layer chromatography. The range of different methods is important due to the destructive nature of some instruments and the number of possible unknown substances that can be found at a scene. Forensic chemists prefer using nondestructive methods first, to preserve evidence and to determine which destructive methods will produce the best results.
Oil analysis (OA) is the laboratory analysis of a lubricant's properties, suspended contaminants, and wear debris. OA is performed during routine predictive maintenance to provide meaningful and accurate information on lubricant and machine condition. By tracking oil analysis sample results over the life of a particular machine, trends can be established which can help eliminate costly repairs. The study of wear in machinery is called tribology. Tribologists often perform or interpret oil analysis data.

Fire investigation, sometimes referred to as origin and cause investigation, is the analysis of fire-related incidents. After firefighters extinguish a fire, an investigation is launched to determine the origin and cause of the fire or explosion. These investigations can occur in two stages. The first stage is an investigation of the scene of the fire to establish its origin and cause. The second step is to conduct laboratory examination on the retrieved samples. Investigations of such incidents require a systematic approach and knowledge of fire science.

Forensic biology is the application of biological principles and techniques in the investigation of criminal and civil cases. Forensic biology is primarily concerned with analyzing biological and serological evidence in order to obtain a DNA profile, which aids law enforcement in the identification of potential suspects or unidentified remains. This field encompasses various sub-branches, including forensic anthropology, forensic entomology, forensic odontology, forensic pathology, and forensic toxicology.
Solid phase microextraction, or SPME, is a solid phase extraction sampling technique that involves the use of a fiber coated with an extracting phase, that can be a liquid (polymer) or a solid (sorbent), which extracts different kinds of analytes from different kinds of media, that can be in liquid or gas phase. The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as equilibrium is reached or, in case of short time pre-equilibrium, with help of convection or agitation.
Dissolved gas analysis (DGA) is an examination of electrical transformer oil contaminants. Insulating materials within electrical equipment liberate gases as they slowly break down over time. The composition and distribution of these dissolved gases are indicators of the effects of deterioration, such as pyrolysis or partial discharge, and the rate of gas generation indicates the severity. DGA is beneficial to a preventive maintenance program.
The following outline is provided as an overview of and topical guide to forensic science:

Forensic geology is the study of evidence relating to materials found in the Earth used to answer questions raised by the legal system.

In fire protection, an accelerant is any substance or mixture that accelerates or speeds the development and escalation of fire. Accelerants are often used to commit arson, and some accelerants may cause an explosion. Some fire investigators use the term "accelerant" to mean any substance that initiates and promotes a fire without implying intent or malice. The accelerant works by burning rapidly. As such, the accelerant itself is consumed in the process, and should not be considered as a catalyst. In Arson investigation, the significance of accelerant is to detect the presence of a such substance in order to proved that the fire is classified as an arson.
The history of chromatography spans from the mid-19th century to the 21st. Chromatography, literally "color writing", was used—and named— in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll and carotenoids. New forms of chromatography developed in the 1930s and 1940s made the technique useful for a wide range of separation processes and chemical analysis tasks, especially in biochemistry.
Headspace gas chromatography uses headspace gas—from the top or "head" of a sealed container containing a liquid or solid brought to equilibrium—injected directly onto a gas chromatographic column for separation and analysis. In this process, only the most volatile substances make it to the column. The technique is commonly applied to the analysis of polymers, food and beverages, blood alcohol levels, environmental variables, cosmetics, and pharmaceutical ingredients.

Forensic firearm examination is the forensic process of examining the characteristics of firearms or bullets left behind at a crime scene. Specialists in this field try to link bullets to weapons and weapons to individuals. They can raise and record obliterated serial numbers in an attempt to find the registered owner of a weapon and look for fingerprints on a weapon and cartridges.

Evidence packaging involves the specialized packaging methods and materials used for physical evidence. Items need to be collected at a crime scene or a fire scene, forwarded to a laboratory for forensic analysis, put in secure storage, and used in a courtroom, all while maintaining the chain of custody. Items might include DNA, drugs, hair samples, body parts, blood samples, sperm, knives, vomit, firearms, bullets, fire accelerants, computers, checkbooks, etc.











