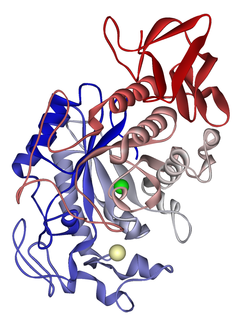
An amylase is an enzyme that catalyses the hydrolysis of starch into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. Specific amylase proteins are designated by different Greek letters. All amylases are glycoside hydrolases and act on α-1,4-glycosidic bonds.
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
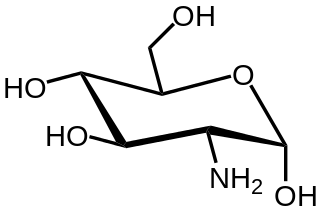
In organic chemistry, an amino sugar is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being N-Acetyl-d-glucosamine, which is the main component of chitin.

Nyctanthes arbor-tristis, also known as the Night-flowering jasmine or Parijat , is a species of Nyctanthes native to South Asia and Southeast Asia.

β-Glucocerebrosidase is an enzyme with glucosylceramidase activity that is needed to cleave, by hydrolysis, the beta-glycosidic linkage of the chemical glucocerebroside, an intermediate in glycolipid metabolism that is abundant in cell membranes. It is localized in the lysosome, where it remains associated with the lysosomal membrane. β-Glucocerebrosidase is 497 amino acids in length and has a molecular weight of 59,700 Daltons.

Antiarins are cardiac glycoside poisons produced by the upas tree. There are two forms, α-antiarin and β-antiarin.
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance.
The Crich β-mannosylation in organic chemistry is a synthetic strategy which is used in carbohydrate synthesis to generate a 1,2-cis-glycosidic bond. This type of linkate is generally very difficult to make, and specific methods like the Crich β-mannosylation are used to overcome these issues.

The flavanonols are a class of flavonoids that use the 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one backbone.
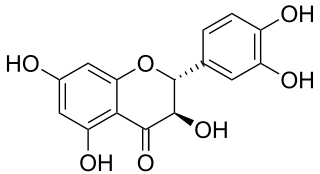
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols.

Afzelechin is a flavan-3-ol, a type of flavonoid. It can be found in Bergenia ligulata. It exists as at least 2 major epimers.

Selliguea feei is a fern belonging to the genus Selliguea in the family Polypodiaceae. This fern can be collected in Indonesia. The species name feei commemorates the botanist Antoine Laurent Apollinaire Fée.

Eupalitin is an O-methylated flavonol. It can be found in Ipomopsis aggregata.
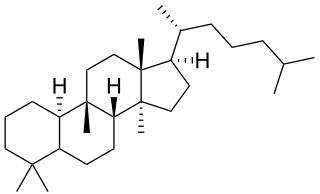
Cucurbitane is a class of chemical compounds with formula C
30H
54. It is a polycyclic hydrocarbon, specifically triterpene. It is also an isomer of lanostane, from which it differs by the formal shift of a methyl group from the 10 to the 9β position in the standard steroid numbering scheme.
A kuguaglycoside is one of several chemical compounds isolated from the roots of the bitter melon vine by J.-C. Chen and others.

Myrica esculenta is a tree or large shrub native to the hills of northern India, southern Bhutan and Nepal. Its common names include box myrtle, bayberry and kaphal. Its berries are edible and are consumed locally. It is the state fruit of the northern Indian state of Uttarakhand.

Geranin A is an A type proanthocyanidin of the propelargonidin sub type. Its structure is epi-afzelechin-(4β→8, 2β→O→7)-afzelechin.

Paullinia pinnata is a flowering plant species in the genus of Paullinia found in South America and Africa.

Teucrium gnaphalodes is a plant species in the genus Teucrium. It is endemic to the Iberian Peninsula and grows at altitudes between 200 and 1500 m. It flowers from March to July.















