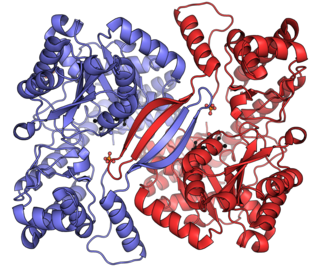
Alanine transaminase (ALT) is a transaminase enzyme. It is also called alanine aminotransferase and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first characterized in the mid-1950s by Arthur Karmen and colleagues. ALT is found in plasma and in various body tissues but is most common in the liver. It catalyzes the two parts of the alanine cycle. Serum ALT level, serum AST level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.

Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase, is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.
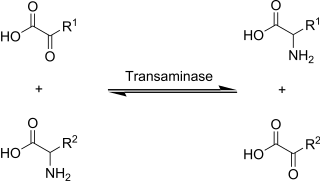
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
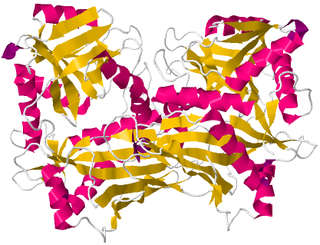
Branched-chain amino acid aminotransferase (BCAT), also known as branched-chain amino acid transaminase, is an aminotransferase enzyme (EC 2.6.1.42) which acts upon branched-chain amino acids (BCAAs). It is encoded by the BCAT2 gene in humans. The BCAT enzyme catalyzes the conversion of BCAAs and α-ketoglutarate into branched chain α-keto acids and glutamate.
The enzyme 2,2-dialkylglycine decarboxylase (pyruvate) (EC 4.1.1.64) catalyzes the chemical reaction
In enzymology, a 2,5-diaminovalerate transaminase is an enzyme that catalyzes the chemical reaction

In enzymology, 4-aminobutyrate transaminase, also called GABA transaminase or 4-aminobutyrate aminotransferase, or GABA-T, is an enzyme that catalyzes the chemical reaction:
In enzymology, an adenosylmethionine-8-amino-7-oxononanoate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, an alanine-oxo-acid transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, an aromatic-amino-acid-glyoxylate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, an aromatic-amino-acid transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a cephalosporin-C transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a D-amino-acid transaminase is an enzyme that catalyzes the chemical reaction:
In enzymology, glutamate-prephenate aminotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a glutamine-pyruvate transaminase is an enzyme that catalyzes the chemical reaction

In enzymology, a kynurenine-oxoglutarate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a leucine transaminase is an enzyme that catalyzes the chemical reaction

In enzymology, a tryptophan transaminase is an enzyme that catalyzes the chemical reaction
Methionine transaminase is an enzyme with systematic name L-methionine:2-oxo-acid aminotransferase. This enzyme catalyses the following chemical reaction








