The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.

The enzyme argininosuccinate lyase (EC 4.3.2.1, ASL, argininosuccinase; systematic name 2-(N ω-L-arginino)succinate arginine-lyase (fumarate-forming)) catalyzes the reversible breakdown of argininosuccinate:

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
The enzyme β-Alanyl-CoA ammonia-lyase (EC 4.3.1.6) catalyzes the chemical reaction

Cystathionine beta-lyase, also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction
The enzyme erythro-3-hydroxyaspartate ammonia-lyase (EC 4.3.1.20) catalyzes the chemical reaction
The enzyme ethanolamine ammonia-lyase (EC 4.3.1.7) catalyzes the chemical reaction
In enzymology, a formimidoyltetrahydrofolate cyclodeaminase (EC 4.3.1.4) is an enzyme that catalyzes the chemical reaction
The enzyme L-serine ammonia-lyase (EC 4.3.1.17) catalyzes the chemical reaction
The enzyme methylaspartate ammonia-lyase (EC 4.3.1.2) catalyzes the chemical reaction
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and trans-cinnamic acid.:
The enzyme threo-3-hydroxyaspartate ammonia-lyase (EC 4.3.1.16) is an enzyme that catalyzes the chemical reaction

The enzyme aspartate 1-decarboxylase (EC 4.1.1.11) catalyzes the chemical reaction

In enzymology, an aspartate 4-decarboxylase (EC 4.1.1.12) is an enzyme that catalyzes the chemical reaction
The enzyme orsellinate decarboxylase (EC 4.1.1.58) catalyzes the chemical reaction
The enzyme tryptophanase (EC 4.1.99.1) catalyzes the chemical reaction
In enzymology, an aspartate—ammonia ligase (EC 6.3.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, an aspartate—ammonia ligase (ADP-forming) (EC 6.3.1.4) is an enzyme that catalyzes the chemical reaction
Fumarate lyase belongs to the lyase class of enzymes. These proteins use fumarate as a substrate. They have been shown to share a short conserved sequence around a methionine which is probably involved in the catalytic activity of this type of enzymes.
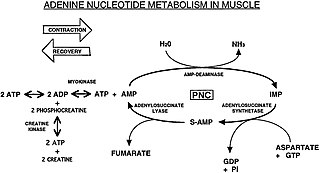
The Purine Nucleotide Cycle is a metabolic pathway in protein metabolism requiring the amino acids aspartate and glutamate. The cycle is used to regulate the levels of adenine nucleotides, in which ammonia and fumarate are generated. AMP coverts into IMP and the byproduct ammonia. IMP converts to S-AMP (adenylosuccinate), which then coverts to AMP and the byproduct fumarate. The fumarate goes on to produce ATP (energy) via oxidative phosphorylation as it enters the Krebs cycle and then the electron transport chain. Lowenstein first described this pathway and outlined its importance in processes including amino acid catabolism and regulation of flux through glycolysis and the Krebs cycle.