Related Research Articles

A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.
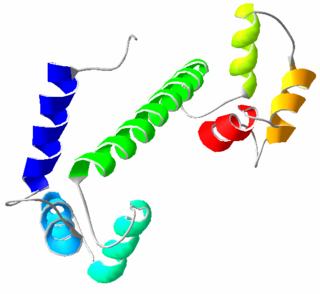
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

The Rossmann fold is a tertiary fold found in proteins that bind nucleotides, such as enzyme cofactors FAD, NAD+, and NADP+. This fold is composed of alternating beta strands and alpha helical segments where the beta strands are hydrogen bonded to each other forming an extended beta sheet and the alpha helices surround both faces of the sheet to produce a three-layered sandwich. The classical Rossmann fold contains six beta strands whereas Rossmann-like folds, sometimes referred to as Rossmannoid folds, contain only five strands. The initial beta-alpha-beta (bab) fold is the most conserved segment of the Rossmann fold. The motif is named after Michael Rossmann who first noticed this structural motif in the enzyme lactate dehydrogenase in 1970 and who later observed that this was a frequently occurring motif in nucleotide binding proteins.

Deubiquitinating enzymes (DUBs), also known as deubiquitinating peptidases, deubiquitinating isopeptidases, deubiquitinases, ubiquitin proteases, ubiquitin hydrolases, ubiquitin isopeptidases, are a large group of proteases that cleave ubiquitin from proteins. Ubiquitin is attached to proteins in order to regulate the degradation of proteins via the proteasome and lysosome; coordinate the cellular localisation of proteins; activate and inactivate proteins; and modulate protein-protein interactions. DUBs can reverse these effects by cleaving the peptide or isopeptide bond between ubiquitin and its substrate protein. In humans there are nearly 100 DUB genes, which can be classified into two main classes: cysteine proteases and metalloproteases. The cysteine proteases comprise ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), Machado-Josephin domain proteases (MJDs) and ovarian tumour proteases (OTU). The metalloprotease group contains only the Jab1/Mov34/Mpr1 Pad1 N-terminal+ (MPN+) (JAMM) domain proteases.

ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling.

An alpha solenoid is a protein fold composed of repeating alpha helix subunits, commonly helix-turn-helix motifs, arranged in antiparallel fashion to form a superhelix. Alpha solenoids are known for their flexibility and plasticity. Like beta propellers, alpha solenoids are a form of solenoid protein domain commonly found in the proteins comprising the nuclear pore complex. They are also common in membrane coat proteins known as coatomers, such as clathrin, and in regulatory proteins that form extensive protein-protein interactions with their binding partners. Examples of alpha solenoid structures binding RNA and lipids have also been described.
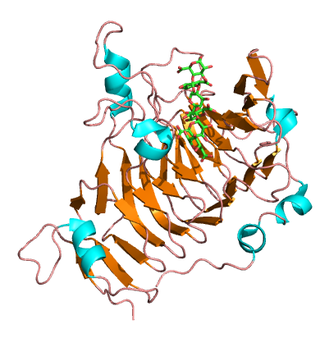
Pectinesterase (EC 3.1.1.11; systematic name pectin pectylhydrolase) is a ubiquitous cell-wall-associated enzyme that presents several isoforms that facilitate plant cell wall modification and subsequent breakdown. It catalyzes the following reaction:

CTGF, also known as CCN2 or connective tissue growth factor, is a matricellular protein of the CCN family of extracellular matrix-associated heparin-binding proteins. CTGF has important roles in many biological processes, including cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair, and is critically involved in fibrotic disease and several forms of cancers.
Oleosins are structural proteins found in vascular plant oil bodies and in plant cells. Oil bodies are not considered organelles because they have a single layer membrane and lack the pre-requisite double layer membrane in order to be considered an organelle. They are found in plant parts with high oil content that undergo extreme desiccation as part of their maturation process, and help stabilize the bodies.

Apetala 2(AP2) is a gene and a member of a large family of transcription factors, the AP2/EREBP family. In Arabidopsis thaliana AP2 plays a role in the ABC model of flower development. It was originally thought that this family of proteins was plant-specific; however, recent studies have shown that apicomplexans, including the causative agent of malaria, Plasmodium falciparum encode a related set of transcription factors, called the ApiAP2 family.
Expansins are a family of closely related nonenzymatic proteins found in the plant cell wall, with important roles in plant cell growth, fruit softening, abscission, emergence of root hairs, pollen tube invasion of the stigma and style, meristem function, and other developmental processes where cell wall loosening occurs. Expansins were originally discovered as mediators of acid growth, which refers to the widespread characteristic of growing plant cell walls to expand faster at low (acidic) pH than at neutral pH. Expansins are thus linked to auxin action. They are also linked to cell enlargement and cell wall changes induced by other plant hormones such as gibberellin, cytokinin, ethylene and brassinosteroids.

AP-1 complex subunit gamma-1 is a protein that in humans is encoded by the AP1G1 gene.
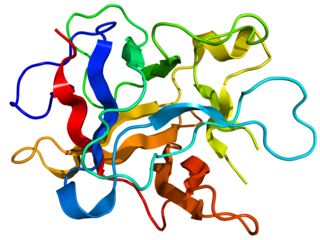
Kunitz soybean trypsin inhibitor is a type of protein contained in legume seeds which functions as a protease inhibitor. Kunitz-type Soybean Trypsin Inhibitors are usually specific for either trypsin or chymotrypsin. They are thought to protect seeds against consumption by animal predators.
The Zona pellucida-like domain is a large protein region of about 260 amino acids. It has been recognised in a variety of receptor-like eukaryotic glycoproteins. All of these molecules are mosaic proteins with a large extracellular region composed of various domains, often followed by either a transmembrane region and a very short cytoplasmic region or by a GPI-anchor.

S-Adenosylmethionine synthetase, also known as methionine adenosyltransferase (MAT), is an enzyme that creates S-adenosylmethionine by reacting methionine and ATP.

In molecular biology, the cyclase-associated protein family (CAP) is a family of highly conserved actin-binding proteins present in a wide range of organisms including yeast, flies, plants, and mammals. CAPs are multifunctional proteins that contain several structural domains. CAP is involved in species-specific signalling pathways. In Drosophila, CAP functions in Hedgehog-mediated eye development and in establishing oocyte polarity. In Dictyostelium discoideum, CAP is involved in microfilament reorganisation near the plasma membrane in a PIP2-regulated manner and is required to perpetuate the cAMP relay signal to organise fruitbody formation. In plants, CAP is involved in plant signalling pathways required for co-ordinated organ expansion. In yeast, CAP is involved in adenylate cyclase activation, as well as in vesicle trafficking and endocytosis. In both yeast and mammals, CAPs appear to be involved in recycling G-actin monomers from ADF/cofilins for subsequent rounds of filament assembly. In mammals, there are two different CAPs that share 64% amino acid identity.

In molecular biology, the GCM transcription factors are a family of proteins which contain a GCM motif. The GCM motif is a domain that has been identified in proteins belonging to a family of transcriptional regulators involved in fundamental developmental processes which comprise Drosophila melanogaster GCM and its mammalian homologues. In GCM transcription factors the N-terminal moiety contains a DNA-binding domain of 150 amino acids. Sequence conservation is highest in this GCM domain. In contrast, the C-terminal moiety contains one or two transactivating regions and is only poorly conserved.

In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria. Bacteria use TAAs in order to infect their host cells via a process called cell adhesion. TAAs also go by another name, oligomeric coiled-coil adhesins, which is shortened to OCAs. In essence, they are virulence factors, factors that make the bacteria harmful and infective to the host organism.

In molecular biology, YadA is a protein domain which is short for Yersinia adhesin A. These proteins have strong sequence and structural homology, particularly at their C-terminal end. The function is to promote their pathogenicity and virulence in host cells, though cell adhesion. YadA is found in three pathogenic species of Yersinia, Y. pestis,Y. pseudotuberculosis, and Y. enterocolitica. The YadA domain is encoded for by a virulence plasmid in Yersinia, which encodes a type-III secretion (T3S) system consisting of the Ysc injectisome and the Yop effectors.

The membrane (M) protein is an integral membrane protein that is the most abundant of the four major structural proteins found in coronaviruses. The M protein organizes the assembly of coronavirus virions through protein-protein interactions with other M protein molecules as well as with the other three structural proteins, the envelope (E), spike (S), and nucleocapsid (N) proteins.
References
- 1 2 Hattori J, Boutilier KA, van Lookeren Campagne MM, Miki BL (September 1998). "A conserved BURP domain defines a novel group of plant proteins with unusual primary structures". Mol. Gen. Genet. 259 (4): 424–8. doi:10.1007/s004380050832. PMID 9790599. S2CID 21359007.
- 1 2 Batchelor AK, Boutilier K, Miller SS, Hattori J, Bowman LA, Hu M, Lantin S, Johnson DA, Miki BL (August 2002). "SCB1, a BURP-domain protein gene, from developing soybean seed coats". Planta. 215 (4): 523–32. doi:10.1007/s00425-002-0798-1. PMID 12172833. S2CID 19185882.
- ↑ Wang A, Xia Q, Xie W, Datla R, Selvaraj G (November 2003). "The classical Ubisch bodies carry a sporophytically produced structural protein (RAFTIN) that is essential for pollen development". Proc. Natl. Acad. Sci. U.S.A. 100 (24): 14487–92. Bibcode:2003PNAS..10014487W. doi: 10.1073/pnas.2231254100 . PMC 283618 . PMID 14612572.