
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. A group of the structure R2C=C=CR− is called allenyl, while a substituent attached to an allene is referred to as an allenic substituent. In analogy to allylic and propargylic, a substituent attached to a saturated carbon α to an allene is referred to as an allenylic substituent. While allenes have two consecutive ('cumulated') double bonds, compounds with three or more cumulated double bonds are called cumulenes.
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs.

In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.
Barrelene is a bicyclic organic compound with chemical formula C8H8 and systematic name bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel, with the staves being three ethylene units attached to two methine groups. It is the formal Diels–Alder adduct of benzene and acetylene. Due to its unusual molecular geometry, the compound is of considerable interest to theoretical chemists.
The alpha effect refers to the increased nucleophilicity of an atom due to the presence of an adjacent (alpha) atom with lone pair electrons. This first atom does not necessarily exhibit increased basicity compared with a similar atom without an adjacent electron-donating atom, resulting in a deviation from the classical Brønsted-type reactivity-basicity relationship. In other words, the alpha effect refers to nucleophiles presenting higher nucleophilicity than the predicted value obtained from the Brønsted basicity. The representative examples would be high nucleophilicities of hydroperoxide (HO2−) and hydrazine (N2H4). The effect is now well established with numerous examples and became an important concept in mechanistic chemistry and biochemistry. However, the origin of the effect is still controversial without a clear winner.

The Achmatowicz reaction, also known as the Achmatowicz rearrangement, is an organic synthesis in which a furan is converted to a dihydropyran. In the original publication by the Polish Chemist Osman Achmatowicz Jr. in 1971 furfuryl alcohol is reacted with bromine in methanol to 2,5-dimethoxy-2,5-dihydrofuran which rearranges to the dihydropyran with dilute sulfuric acid. Additional reaction steps, alcohol protection with methyl orthoformate and boron trifluoride) and then ketone reduction with sodium borohydride produce an intermediate from which many monosaccharides can be synthesised.
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen.

Zoltan George Hajos was a Hungarian-American organic chemist. Originally an academic in his native Budapest, then an industrial chemist in the pharmaceutical industry, he is known for the Hajos–Parrish–Eder–Sauer–Wiechert reaction.

Anthony Gerard Martin Barrett FRS, FMedSci is a British chemist, and Sir Derek Barton Professor of Synthesis, Glaxo Professor of Organic Chemistry at Imperial College London. He is Director of the Wolfson Centre for Organic Chemistry in Medical Science. He was elected a fellow of the Royal Society in 1999 and Academy of Medical Sciences in 2003. He obtained a BSc as well as PhD from Imperial College London in 1973 and 1975 respectively.
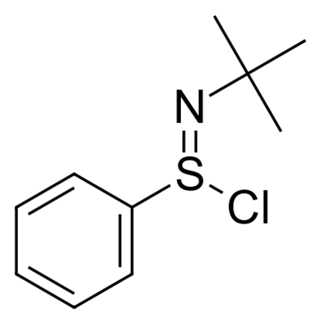
N-tert-Butylbenzenesulfinimidoyl chloride is a useful oxidant for organic synthesis reactions. It is a good electrophile, and the sulfimide S=N bond can be attacked by nucleophiles, such as alkoxides, enolates, and amide ions. The nitrogen atom in the resulting intermediate is basic, and can abstract an α-hydrogen to create a new double bond.
The Davis–Beirut reaction is N,N-bond forming heterocyclization that creates numerous types of 2H-indazoles and indazolones in both acidic and basic conditions The Davis–Beirut reaction is named after Mark Kurth and Makhluf Haddadin's respective universities; University of California, Davis and American University of Beirut, and is appealing because it uses inexpensive starting materials and does not require toxic metals.

MoOPH, also known as oxodiperoxymolybdenum(pyridine)-(hexamethylphosphoric triamide), is a reagent used in organic synthesis. It contains a molybdenum(VI) center with multiple oxygen ligands, coordinated with pyridine and HMPA ligands. It is an electrophilic source of oxygen that reacts with enolates and related structures, and thus can be used for alpha-hydroxylation of carbonyl-containing compounds. Other reagents used for alpha-hydroxylation via enol or enolate structures include Davis oxaziridine, oxygen, and various peroxyacids. This reagent was first utilized by Edwin Vedejs as an efficient alpha-hydroxylating agent in 1974 and an effective preparative procedure was later published in 1978.
Mark S. Cushman is an American chemist, whose primary research is in the area of medicinal chemistry. He completed his pre-pharmacy studies at Fresno State College (now California State University, Fresno) in 1965. He then attended the University of California San Francisco (as a University of California Regents Scholar), earning a Pharm.D. in 1969 and a Ph.D. in Medicinal Chemistry in 1973. Thereafter, he performed postdoctoral training in the laboratory of George Büchi, Ph.D., at the Massachusetts Institute of Technology (MIT). There, his research focused on the discovery and development of new synthetic methodologies, and the isolation and structural characterization of mycotoxins from Aspergillus niger. In 1975, he joined the Department of Medicinal Chemistry and Molecular Pharmacology (at the time, Department of Medicinal Chemistry and Pharmacognosy) at Purdue University. From 1983 to 1984, Prof. Cushman was a Senior Fulbright Scholar at Munich Technical University working in the laboratory of Professor Adelbert Bacher. His sabbatical work dealt with the design and synthesis of probes to elucidate key aspects of the biosynthesis of riboflavin (vitamin B2). Currently he holds the rank of Distinguished Professor Emeritus of Medicinal Chemistry at Purdue University. He has mentored 40 graduate students, 59 postdoctoral researchers, and 5 visiting scholars. He has published 348 papers and holds 41 patents. His work has ~17,000 citations with an h-index of 69. His most cited papers had 471, 403, and 299 citations as of August 2021. He has made seminal contributions to the fields of synthetic and medicinal chemistry including the development of new synthetic methodologies, the synthesis of natural products, and the preparation of antivirals, antibacterials, and anticancer agents, and mechanism probes to understand the function of over thirty macromolecular targets. One of his main scientific contributions is the development of the indenoisoquinolines, molecules that inhibit the action of toposiomerase I (Top1) and stabilize the G-quadruplex in the Myc promoter. Three indenoisoquinolines designed and synthesized by his research group at Purdue University [indotecan (LMP 400), indimitecan (LMP 776), and LMP 744] demonstrated potent anticancer activity in vivo and have completed phase I clinical trials at the National Institutes of Health.
F. Dean Toste is the Gerald E. K. Branch Distinguished Professor of Chemistry at the University of California, Berkeley and faculty scientist at the chemical sciences division of Lawrence Berkeley National Lab. He is a prominent figure in the field of organic chemistry and is best known for his contributions to gold chemistry and asymmetric ion-pairing catalysis. Toste was elected a member of the National Academy of Sciences in 2020, and a member of the American Academy of Arts and Sciences in 2018.
Cross dehydrogenative coupling (also known as CDC reaction), coined by Chao-Jun Li of McGill University, is a type of coupling reaction allowing the construction of a carbon–carbon bond or C-Heteroatom bond directly from C-H bonds in the presence of an oxidant, leading to the thermodynamically unfavorable formal removal of a H2 molecule. As such, CDC are couplings belonging to the C-H activation strategy.
In organic chemistry, methylenation is a chemical reaction that inserts a methylene group into a chemical compound:

The nitro-Mannich reaction is the nucleophilic addition of a nitroalkane to an imine, resulting in the formation of a beta-nitroamine. With the reaction involving the addition of an acidic carbon nucleophile to a carbon-heteroatom double bond, the nitro-Mannich reaction is related to some of the most fundamental carbon-carbon bond forming reactions in organic chemistry, including the aldol reaction, Henry reaction and Mannich reaction.

Benjamin List is a German chemist who is one of the directors of the Max Planck Institute for Coal Research and professor of organic chemistry at the University of Cologne. He co-developed organocatalysis, a method of accelerating chemical reactions and making them more efficient. He shared the 2021 Nobel Prize in Chemistry with David MacMillan "for the development of asymmetric organocatalysis".
The ketimine Mannich reaction is an asymmetric synthetic technique using differences in starting material to push a Mannich reaction to create an enantiomeric product with steric and electronic effects, through the creation of a ketimine group. Typically, this is done with a reaction with proline or another nitrogen-containing heterocycle, which control chirality with that of the catalyst. This has been theorized to be caused by the restriction of undesired (E)-isomer by preventing the ketone from accessing non-reactive tautomers. Generally, a Mannich reaction is the combination of an amine, a ketone with a β-acidic proton and aldehyde to create a condensed product in a β-addition to the ketone. This occurs through an attack on the ketone with a suitable catalytic-amine unto its electron-starved carbon, from which an imine is created. This then undergoes electrophilic addition with a compound containing an acidic proton. It is theoretically possible for either of the carbonyl-containing molecules to create diastereomers, but with the addition of catalysts which restrict addition as of the enamine creation, it is possible to extract a single product with limited purification steps and in some cases as reported by List et al.; practical one-pot syntheses are possible. The process of selecting a carbonyl-group gives the reaction a direct versus indirect distinction, wherein the latter case represents pre-formed products restricting the reaction's pathway and the other does not. Ketimines selects a reaction group, and circumvent a requirement for indirect pathways.









