
Hydrazones are a class of organic compounds with the structure R
1R
2C=NNH
2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH
2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.

An imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way.
In the Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.

An iminium cation in organic chemistry is a functional group with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.
Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst. The metal is supported on activated carbon to maximize its surface area and activity.
The Leuckart reaction is the chemical reaction that converts aldehydes or ketones to amines by reductive amination in the presence of heat. The reaction, named after Rudolf Leuckart, uses either ammonium formate or formamide as the nitrogen donor and reducing agent. It requires high temperatures, usually between 120 and 130 °C; for the formamide variant, the temperature can be greater than 165 °C.
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent.
The Buchwald–Hartwig amination is a chemical reaction used in organic chemistry for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods for the synthesis of aromatic C–N bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods while significantly expanding the repertoire of possible C–N bond formation.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
The Béchamp reduction is a chemical reaction that converts aromatic nitro compounds to their corresponding anilines using iron as the reductant.

1-Phenylethylamine is the organic compound with the formula C6H5CH(NH2)CH3. Classified as a monoamine, this colorless liquid is often used in chiral resolutions. Like benzylamine, it is highly basic and forms stable ammonium salts and imines.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.

Benzyl carbamate is the organic compound with the formula C6H5CH2OC(O)NH2. The compound can be viewed as the ester of carbamic acid (O=C(OH)(NH2)) and benzyl alcohol, although it is produced from benzyl chloroformate with ammonia. It is a white solid that is soluble in organic solvents and moderately soluble in water. Benzyl carbamate is used as a protected form of ammonia in the synthesis of primary amines. After N-alkylation, C6H5CH2OC(O) group is removable with Lewis acids.
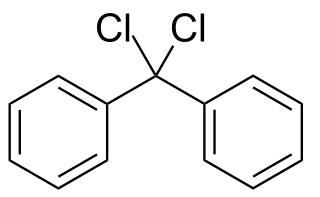
Diphenyldichloromethane is an organic compound with the formula (C6H5)2CCl2. It is a colorless solid that is used as a precursor to other organic compounds.

Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral.

Acetone imine, or 2-propanimine is an organic compound and an imine with the chemical formula (CH3)2CNH. It is a volatile and flammable liquid at room temperature. It is the simplest ketimine. This compound is mainly of academic interest.
In organic chemistry, the Lombardo methylenation is a name reaction that allows for the methylenation of carbonyl compounds with the use of Lombardo's reagent, which is a mix of zinc, dibromomethane, and titanium tetrachloride.











